During the VAD UPDATES sub-session, Dr. Chen presented the latest research and development and clinical progress in CH-VAD and BrioVAD, the fully magnetically levitated left ventricular assist system independently developed by CH Biomedical. His presentation attracted significant attention and rave review from the global community.

Dr. Chen’s Presentation at EUMS 2024
During his presentation, Dr. Chen detailed the original development process of the company’s core products, CH-VAD and BiroVAD (Figure 2, Figure 3), highlighting that the initial prototypes were finalized in 2011 and introduced at the annual meeting of the American Society for Artificial Internal Organs (ASAIO). The products underwent several multidisciplinary optimization iterations before finalizing their design in 2015. CH-VAD and BiroVAD boast a range of globally pioneering technologies in, among others, magnetic levitation structure, hydrodynamic design, and percutaneous driveline design. They also indicate international leadership in key performance indicators for evaluating LVAD, including hemocompatibility, hemodynamics, implant invasiveness, anti-infectivity, as well as portability and reliability (Figure 4 to Figure 6).
.png)
Figure 2
.png)
Figure 3
.png)
.png)
.png)
As an internationally renowned expert in magnetically levitated VAD, Dr. Chen focused on the technical standards of full magnetic levitation during his presentation. He noted that the designs comply with the internationally recognized ISO 14839-3 standard for mechanical vibration, which requires a stability margin assessment for the vibration of rotating machinery equipped with active magnetic bearings (AMBs). He also believed that quality standards be set for magnetic levitation design in the field of VAD to eliminate arbitrary choice and explained that the rotor of a totally magnetically levitated blood pump should be able to levitate in the air and resist tilting movement when rotating.
.png)
The peak angular velocity is 12.57 rad/sec, corresponding to the rotating speed of the upper body of a professional golfer during a swing process.
Dr. Chen also disclosed key outcomes in the clinical application of CH-VAD in China. By September 1st, 2024, the product had been used in 314 patients in China through active promoting internationally advanced concepts of full-process management and multidisciplinary cooperation and by observing the regulatory requirements for post-marketing visit. According to Kaplan-Meier survival analysis, 1-year, 2-year, and 3-year survival rates of the patients are 87.2%, 85.2%, and 82.4%, respectively (Figure 8), higher than data from large international clinical trials and INTERMACS while the incidence of key adverse events, such as stroke (Figure 9), gastrointestinal bleeding (Figure 10), and percutaneous driveline infection (Figure 11) are lower than those in large international clinical trials and INTERMACS.
.png)
Figure 8: Kaplan-Meier Survival
.png)
Figure 9: Freedom from first stroke
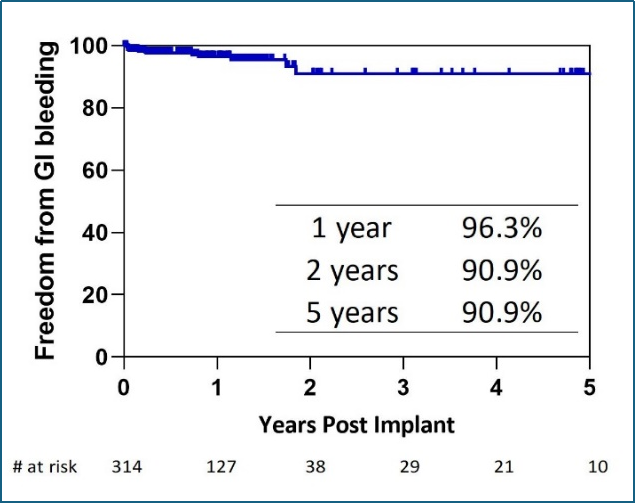
Figure 10: Freedom from first GI bleeding
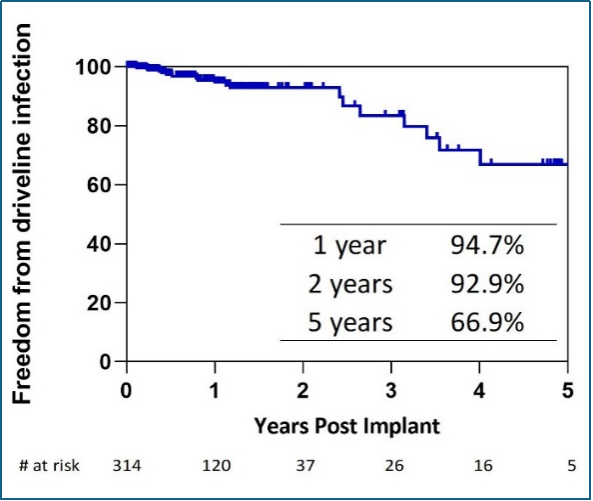
Figure 11: Freedom from first driveline infection
Furthermore, Dr. Chen introduced the upcoming INNOVATE clinical study design and plans for BrioVAD in the United States. This study will employ a randomized controlled approach to evaluate the efficacy and safety of BrioVAD in treating refractory heart failure.
During the summit, Dr. Chen and Mr. Karl Nelson engaged in extensive exchanges and discussions with international leaders of the VAD industry.
.jpg)
In 2020, CH Biomedical became the first Chinese company specializing in implantable VAD to be invited to EUMS and has since consistently reported on the development and application of its products at the conference. In November 2021, CH-VAD, independently developed by CH Biomedical, became China’s first domestically developed LVAD approved by NMPA, marking a new era in the treatment of advanced heart failure in the country. In February 2024, BrioVAD obtained IDE approval from the U.S. FDA, becoming China’s first active implantable medical device approved by the FDA for access to clinical trials. Leveraging its international leadership in key performance indicators, CH Biomedical has achieved successive milestones in China and the United States, further accelerating its expansion into European and other global markets.
On August 7, People’s Daily published an article titled Domestic Fully Magnetically Levitated VAD Serving Patients, commending CH Biomedical for breaking the international technological monopoly for developing a domestically produced, fully magnetically levitated VAD with independent intellectual property rights. The device is currently available in nearly 40 hospitals across China, enabling over 140 patients with advanced heart failure to embark on a “new life”.
Recently, at the 2022 China Medical Development Conference—widely regarded as the country’s premier medical forum—the Chinese Academy of Medical Sciences (CAMS) released two lists: China’s Major Medical Achievements of the 21st Century and China’s Important Medical Advancements in 2021. As a global innovator in the field of Ventricular Assist Devices (VADs), CH Biomedical’s independently developed CH-VAD (NMPA (A) 20213120987) was included in China’s Important Medical Advancements in 2021.
On November 24th 2021, the implantable CH-VAD® left ventricular assist system (CH-VAD® LVAS) of CH Biomedical was approved by the National Medical Products Administration (NMPA) for marketing (Registration No.: GXZZ 20213120987). CH-VAD LVAS was developed independently by CH Biomedical, it is the first Left Ventricular Assist Device (LVAD) with complete independent intellectual property rights approved by NMPA in China, and the first full magnetic levitation LVAD approved by NMPA.


