



Heart failure (HF) is a clinical syndrome caused by structural and/or functional cardiac abnormalities, leading to reduced cardiac output and/or elevated intracardiac pressures at rest or under stress. It is characterized by typical symptoms such as dyspnea, ankle swelling, and fatigue, which may be accompanied by signs such as elevated jugular venous pressure, pulmonary crackles and peripheral edema.1
HF affects a large and growing population worldwide. It is estimated that over 26 million people globally are living with HF,² including 13.7 million in China³ and more than 6.2 million in the United States.⁴ The disease often follows a progressive course that is difficult to reverse with currently available pharmacologic treatments or conventional medical devices, and its prognosis is comparable to or worse than that of many malignant cancers.Heart transplantation remains the standard of care for patients with advanced HF. However, the extreme shortage of donor hearts significantly limits its accessibility.
Ventricular Assist Devices (VADs), as part of mechanical circulatory support (MCS) therapy, have emerged as a key treatment option for patients with advanced HF. In developed countries, VADs have become widely accepted and are now included in clinical guidelines as an effective therapy for refractory HF. This novel, accessible therapy offers a realistic means of significantly extending survival and improving quality of life for patients with advanced HF.
globally
in China
in the United States
Currently, the number of patients with advanced heart failure far exceeds the availability of donor hearts for transplantation. Ventricular assist devices (VADs) not only offer life-saving support, but also provide patients with the opportunity to maintain a normal life.
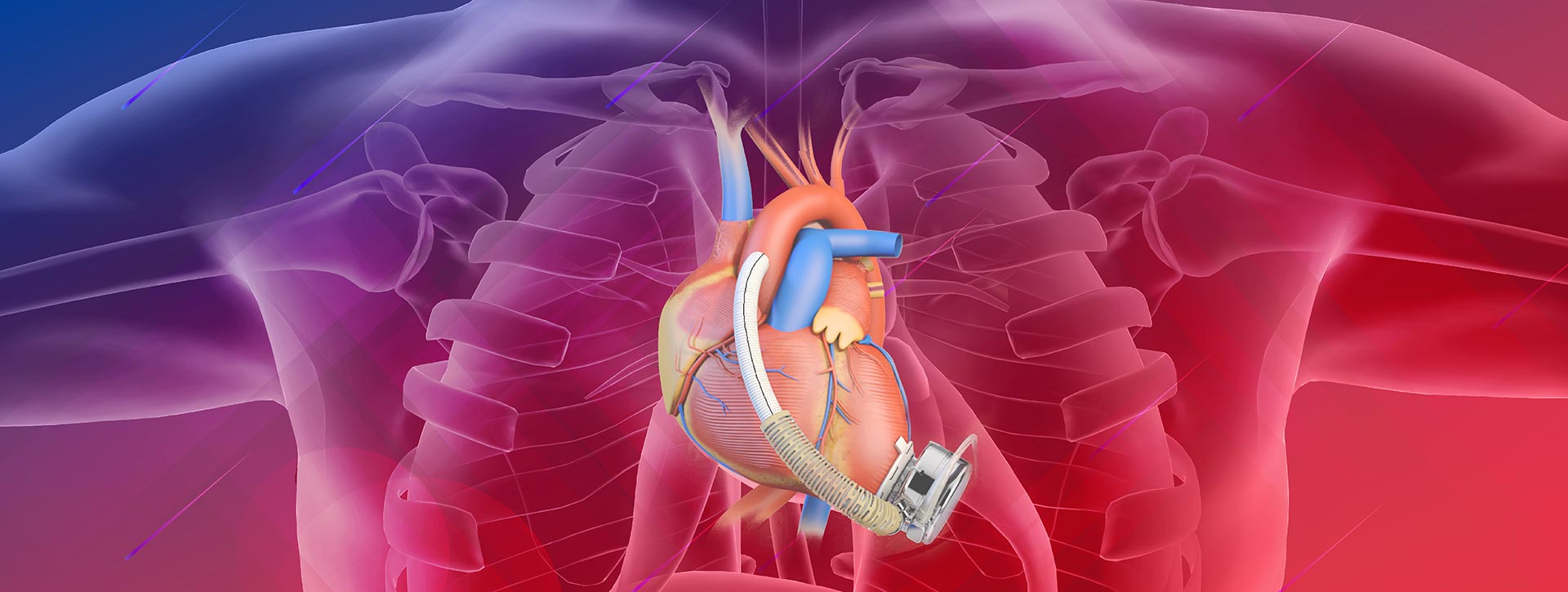
A ventricular assist device (VAD) is a mechatronics device designed to partially or fully replace the heart's pumping function and maintain systemic circulation in patients with heart failure. Its core component is a pump, which draws blood from the ventricle, increases its pressure, and delivers it to the ascending aorta. This process unloads the natural heart, allowing it to rest, and compensates for its reduced ability to pump blood effectively.
After more than five decades of research and development in the United States and other developed countries, VADs were approved by the U.S. FDA in the early 21st century as permanent implantable alternatives to heart transplantations for patients with advanced heart failure. So far, nearly 100,000 clinical implantations have demonstrated that VADs can significantly improve quality of life, prolong survival, and in some cases, even promote myocardial recovery.
Currently, VADs are used in three main clinical scenarios:
Bridge to transplantation (BTT): Supporting patients while they await a suitable donor heart.
Bridge to recovery (BTR): Providing temporary circulatory support for patients with acute heart failure until cardiac function is restored.
Destination therapy (DT): Offering long-term circulatory support for patients with advanced heart failure.

VADs are complex and sophisticated medical devices, first developed in the 1990s. The pump, the core component of a VAD, is responsible for maintaining systemic blood circulation. Based on the pumping mechanism, VADs are categorized into pulsatile flow VADs and continuous-flow (rotary) VADs. Rotary VADs can be further classified by rotor bearing type into contact-bearing VADs (sliding bearings), hydrodynamic or magnetohydrodynamic bearing VADs, and fully magnetically levitated VADs. The evolution from pulsatile to the latest-generation fully magnetically levitated devices represents continuous advancements in the field, aiming for smaller device sizes, enhanced hemocompatibility, reduced adverse event rates, and improved long-term quality of life for patients.


