
On November 25th 2021, the implantable CH-VAD® left ventricular assist system (CH-VAD® LVAS) of CH Biomedical was approved by the National Medical Products Administration (NMPA) for marketing (Registration No.: GXZZ 20213120987). CH-VAD® LVAS was developed independently by CH Biomedical, it is the first Left Ventricular Assist Device (LVAD) with complete independent intellectual property rights approved by NMPA in China, and the first full magnetic levitation LVAD approved by NMPA. This milestone begins commercialization of the new generation LVAD with advanced full magnetic levitation technology in China, opening a new era in heart failure treatment.
Dr. Chen Chen, founder and CEO of the company, said: "Today's ventricular assist devices are not only to save lives, but also to make patients return to their normal and energetic lives. CH Biomedical is committed to lead the advancement of VAD technology globally. CH-VAD® rooted and grew up in the soil of scientific and technological innovation fertilized by strong government. encouragement and support. Therefore, we will first dedicate it to our compatriots in the motherland."
CH-VAD® is the first LVAD with complete independent intellectual property rights approved through the "Special Approval Procedure for Innovative Medical Devices" in China. NMPA commented on this approval: "The core technology of this product is a full magnetic levitation blood pump technology. At present, it has obtained a number of patents in China and the United States. It is the pioneering medical device in implantable Mechanical Circulatory Support (MCS) field in China. Compared with same category products world widely, its key performance indicators have reached the same level, the pump size is smaller, and the implantation invasiveness is much less. This product can meet the current medical demand in heart failure disease treatment with surgical implantable medical device, providing vast benefit to all patients with advanced heart failure.’’
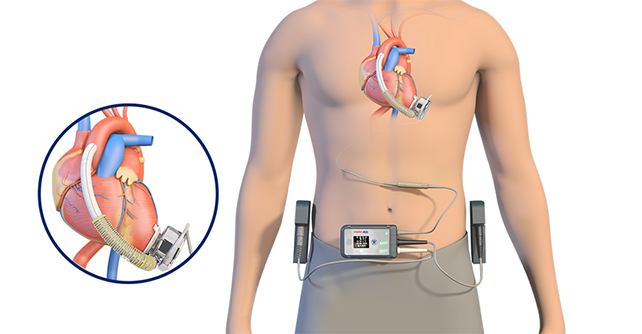
CH-VAD® implantable left ventricular assist system
The implantable CH-VAD® left ventricular assist system is composed of implantable components, external carrying components, peripheral components and surgical tools. It is an electromechanical device used to replace the heart function partially to complete its pumping function and maintain human blood circulation. The core component is a blood pump, which pumps blood out of the heart, increases the pressure, and then sends blood to the aorta, so as to achieve the function of unloading the load of the natural heart, relax the heart, and solve the issue of insufficient blood pumping capacity in the natural heart. It is mainly used to treat patients with end-stage heart failure, providing hemodynamic support for those with advanced refractory left ventricular heart failure.
As the "pearl on the crown" of cardiovascular disease treatment field, implantable VAD applies most advanced technology with a very extensive disciplinary span and technical comprehensiveness. CH-VAD® employs a new generation of full magnetic levitation technology, by properly integrating the magnetic levitation design and flow path design in the pump, multidisciplinary design optimization was achieved, thus forming a comprehensive and original blood pump design without mechanical or hydrodynamics rotor bearing, therefore improved hemocompatibility and reduced related adverse events. CH Biomedical possess clear independent intellectual property rights of CH-VAD® from basic technology to upward construction. Relevant basic technologies have been protected by international patent laws, patents were registered in the United States, Europe, and Japan, which has laid a solid foundation for global marketing in the future.
In terms of clinical investigation, the registered clinical trial of implantable CH-VAD® left ventricular assist system was completed by academician Hu Shengshou from Fuwai Hospital Chinese Academy of Medical Sciences (CAMS) as the Principal Investigator (PI) with the participation of Professor Nianguo Dong from Union Hospital Tongji Medical College Huazhong University of Science and Technology and Professor Zhaoyun Cheng from Fuwai Central China Cardiovascular Hospital. As early as June 2017, Fuwai Hospital successfully completed the First In Human (FIH) application of implantable CH-VAD® left ventricular assist device in China for humanitarian use. The first patient supported with the implanted device has been back to normal life for four and a half years, keeping a good quality of life. From the date of the official clinical trial being launched in March 2019 till today (November 25, 2021) the survival rate of CH-VAD® support has shown great chance to reach at least the survival rate of same type product, compared with the real world data of the latest generation of ventricular assist device in the United States. Among the related adverse events, the most important type was hemocompatibility related adverse events, including intra pump thrombosis, stroke, and gastrointestinal bleeding. As of today (November 25, 2021), no adverse events such as pump thrombosis, stroke and gastrointestinal bleeding have occurred in all patients receiving CH-VAD® implantation. The results of the clinical trial show that CH-VAD® has great advantages in key performance indicators, such as hemocompatibility, surgical invasiveness, anti-infection, system reliability, portability and others.
CH Biomedical’s R&D strength comes from a strong professional team. Since its incorporation, CH Biomedical has gathered a group of VAD experts from domestic and abroad. The core members of the founding team have more than 25 years of VAD R&D experience and rich international vision. With the CH-VAD® leading the industry in key performance indicators internationally, it has become a benchmark for the technological development of the industry, and CH Biomedical has won a good credit as a technological breakthrough seeker among international peers.
Dr. Frank Lin, Chief Technology Officer (CTO) and the President of US subsidiary, said: "CH-VAD® is a model of combining medical science and engineering technology to provide optimized treatment for patients with end-stage heart failure through sincere cooperation of professional teams from domestic and abroad. With its unique and clear technical advantages and excellent performance in the domestic clinical trial, we are full of confidence and expectation for the application and expansion in the world, especially in European and American markets in the future."
At present, China's VAD market is still a blue ocean, and an explosive growth is expected. It is believed that CH-VAD® commercialization process will bring great social influence and economic benefits, and its growing commercial application will save more lives of patients with end-stage heart failure. CH Biomedical will always be committed to lead the advancement of VAD technology and service improvement globally, so that more patients with end-stage heart failure and their families can enjoy a healthy and happy life again.
On August 7, People’s Daily published an article titled Domestic Fully Magnetically Levitated VAD Serving Patients, commending CH Biomedical for breaking the international technological monopoly for developing a domestically produced, fully magnetically levitated VAD with independent intellectual property rights. The device is currently available in nearly 40 hospitals across China, enabling over 140 patients with advanced heart failure to embark on a “new life”.
Recently, at the 2022 China Medical Development Conference—widely regarded as the country’s premier medical forum—the Chinese Academy of Medical Sciences (CAMS) released two lists: China’s Major Medical Achievements of the 21st Century and China’s Important Medical Advancements in 2021. As a global innovator in the field of Ventricular Assist Devices (VADs), CH Biomedical’s independently developed CH-VAD (NMPA (A) 20213120987) was included in China’s Important Medical Advancements in 2021.
On November 24th 2021, the implantable CH-VAD® left ventricular assist system (CH-VAD® LVAS) of CH Biomedical was approved by the National Medical Products Administration (NMPA) for marketing (Registration No.: GXZZ 20213120987). CH-VAD LVAS was developed independently by CH Biomedical, it is the first Left Ventricular Assist Device (LVAD) with complete independent intellectual property rights approved by NMPA in China, and the first full magnetic levitation LVAD approved by NMPA.


