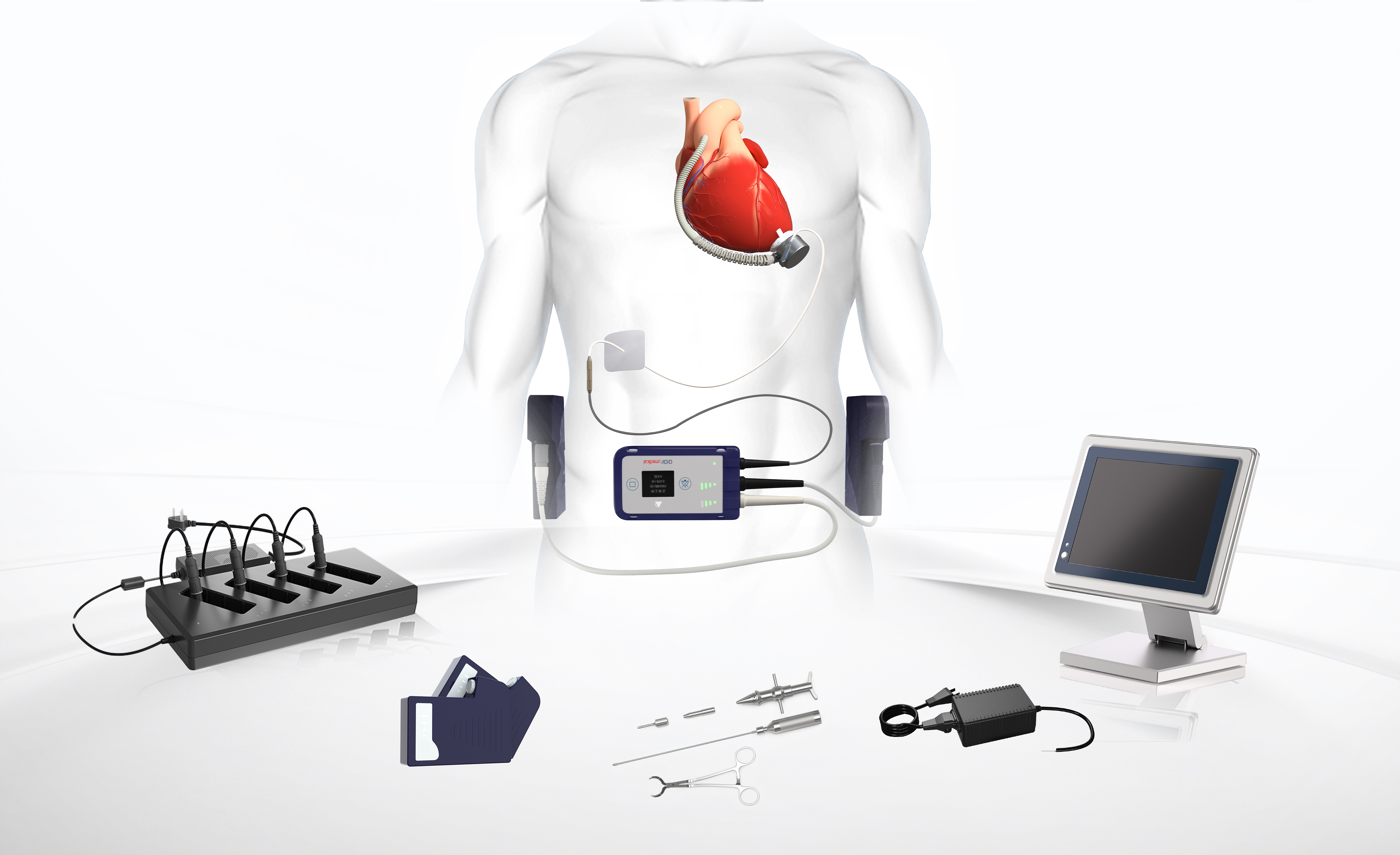
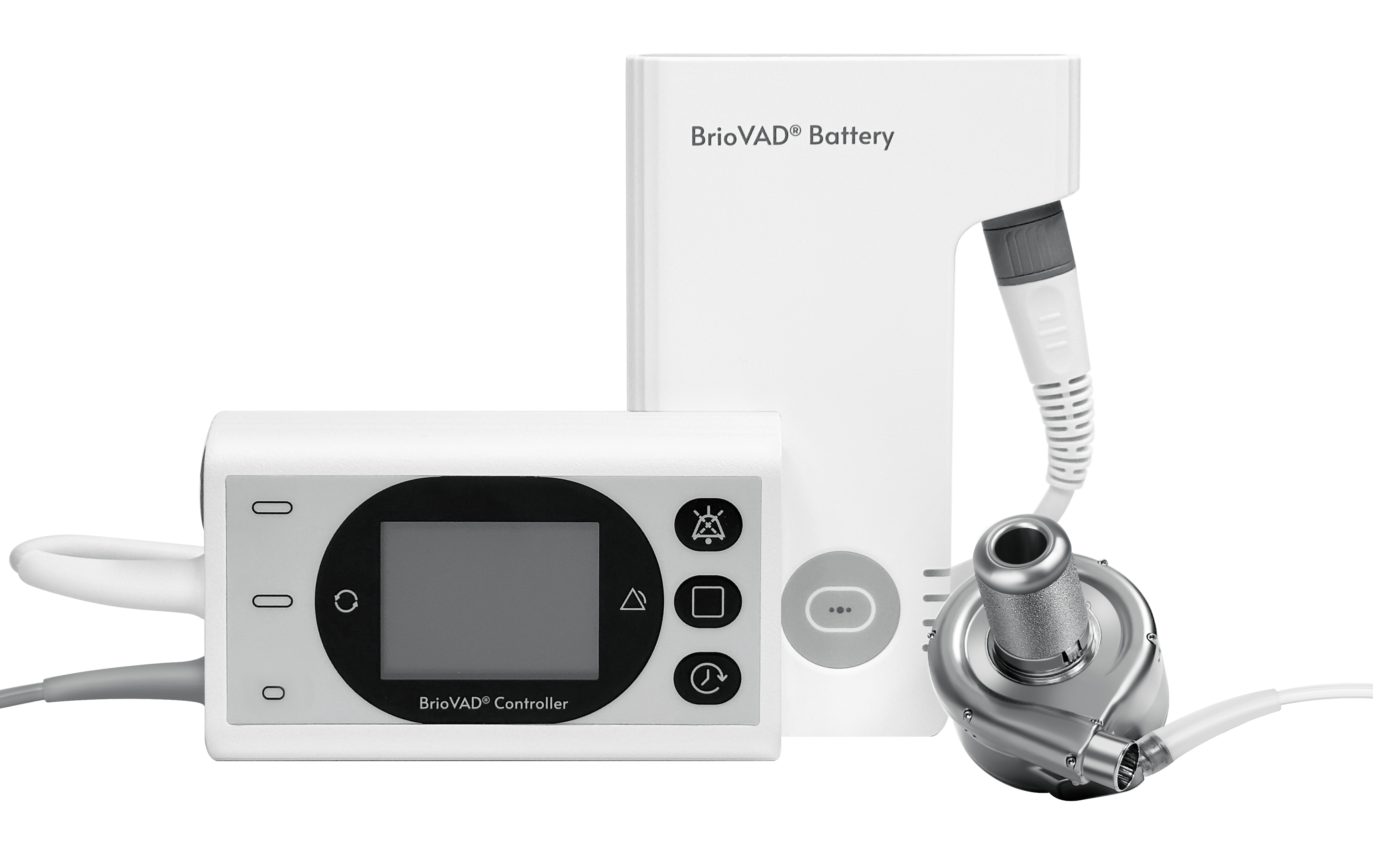
CH Biomedical, Inc.(CHB) was founded in 2008 by a team of scientific and technological experts with overseas experience with the goal of bringing improved survival and quality of life to end-stage heart failure patients. The headquarters of CH Biomedical is located in Suzhou Industrial Park, while the wholly-owned subsidiary, CH Biomedical USA, Inc., is in LA, California. CH Biomedical focuses on the research and development of the world’s state-of-the art ventricular assist device (VAD), promotes the commercialization of the VADs, and brings benefits to patients with end-stage heart failure.
Ventricular assist device (VAD) represents the scientific and technological level of a country's high-end medical devices. It integrates many cutting-edge technologies and advanced sciences in the fields of biomedical engineering such as mechanical design, software control, integrated circuit, and fluid dynamics, as well as experience in the development, management, and manufacturing of high-end medical devices. After more than 10 years of independent research and development in the field of VAD, Our company has developed a new generation of LVAD CH-VAD® with full magnetic levitation technology, and continues to show its strength as a global technology leader in this emerging segment. CHB has completed its initial clinical trial in China. Plans are underway to expand global access to CHB’s lifesaving technology and next-generation maglev CH-VAD® in the United States and other countries.
Adhering to the core values of “Integrity, Truth, Respect, Excellence”, CHB is committed to leading the global artificial heart technology advancement and service improvement, so that more patients with advanced heart failure and their families can enjoy a healthy and happy life again.
To become a global leading science and technology company in advancement of VAD research and development.

To enable more patients with heart failure and their families to enjoy a healthy and happy life again.