Recently, the landmark INNOVATE clinical trial of CH Biomedical’s BrioVAD has been approved for Medicare coverage by the U.S. Centers for Medicare & Medicaid Services (CMS). Under this approval, the required medical device, along with its associated routine examinations and services will be reimbursed by Medicare.
As a federal agency under the U.S. Department of Health and Human Services, CMS administers Medicare, Medicaid and the Children's Health Insurance Program (CHIP). Since 1995, CMS has collaborated with the U.S. Food and Drug Administration (FDA) to establish a framework that facilitates Medicare coverage for Investigational Device Exemption (IDE) studies. Based on the risk level and clinical research foundation of relevant studies, Medicare may cover the associated costs of IDE studies. This initiative ensures that patients can participate in clinical trials without financial constraints while supporting innovative medical device companies in generating revenue and fostering continued investment in medical device research and development.
According to Medicare coverage guidelines, the INNOVATE Trial is classified under MS-DRG 001, which includes reimbursement for BrioVAD, the implantable left ventricular assist system, as well as its related routine examinations and service fees, with an average Medicare payment of approximately $220,000 per patient.
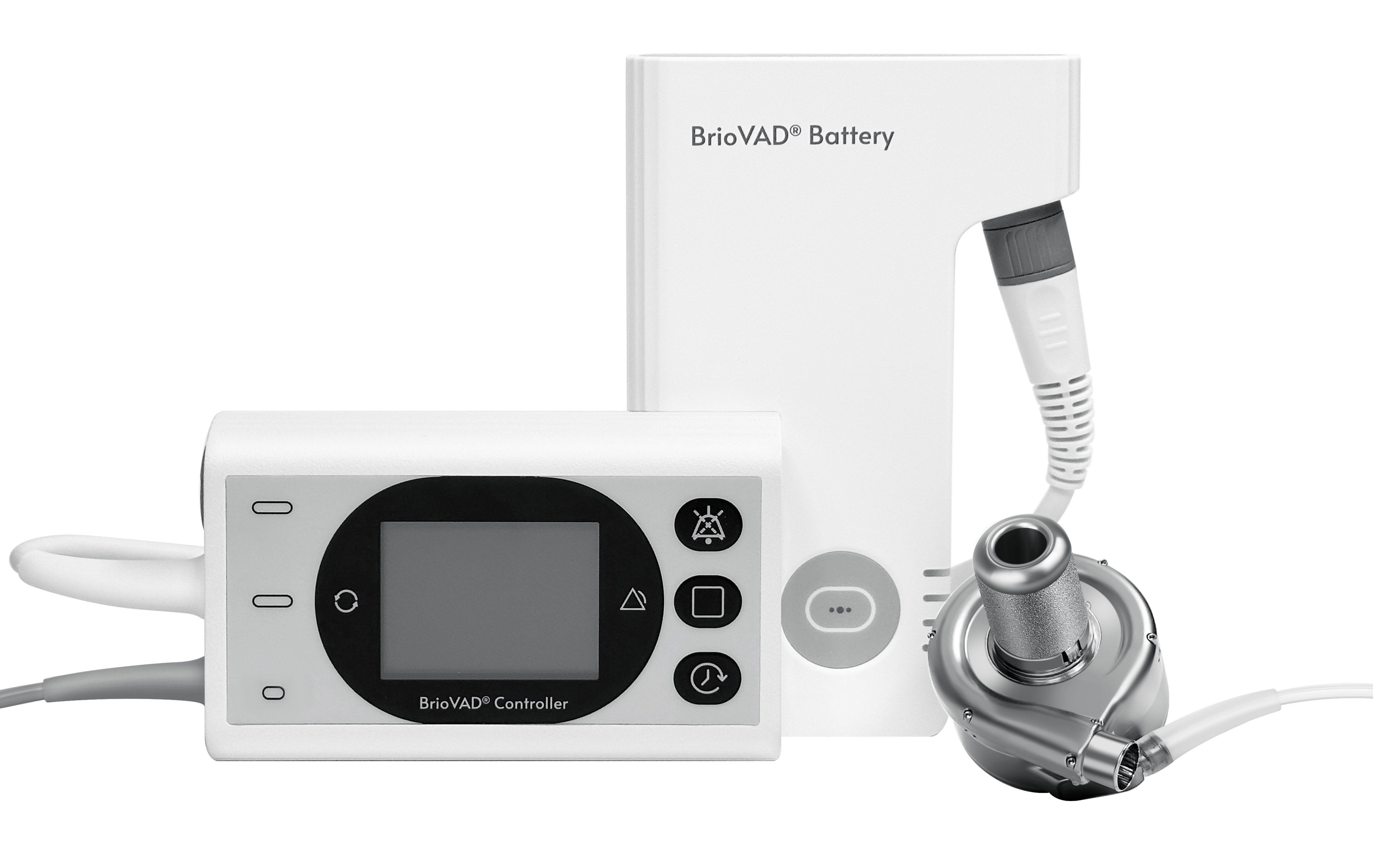
The BrioVAD System
The INNOVATE Trial is designed to evaluate the efficacy and safety of the BrioVAD system, a novel fully magnetically levitated left ventricular assist system independently developed by CH Biomedical, in treating advanced, refractory heart failure. The study adopts a randomized, controlled protocol to compare BrioVAD against the previously FDA-approved LVAS. The BrioVAD is designed to provide short- and long-term circulatory support for patients with advanced heart failure. Based on the excellent hemocompatibility of the CH-VAD, BrioVAD introduces multiple technological innovations to enhance portability of external wearable components while further optimizing overall system performance.
The study was approved by the FDA in the first quarter of 2024, making it as China’s first original active implantable medical device to receive FDA approval for a U.S. clinical trial. Currently, CH Biomedical is expediting preparations for the clinical trial in the U.S.
.png)
First Investigator Meeting for the INNOVATE Trial
The United States represents the largest single market for the LVAD industry globally and is the first key overseas market in CH Biomedical’s international expansion strategy. The CMS’s approval of Medicare reimbursement for the INNOVATE Trail eliminates financial barriers for patient enrollment, facilitating recruitment for the clinical trial. Additionally, this approval further validates the scientific rigor and feasibility of the study. This milestone will accelerate the progress of the clinical trial in the U.S., drive rapid revenue growth, and provide strong momentum for CH Biomedical’s global commercialization strategy.
On August 7, People’s Daily published an article titled Domestic Fully Magnetically Levitated VAD Serving Patients, commending CH Biomedical for breaking the international technological monopoly for developing a domestically produced, fully magnetically levitated VAD with independent intellectual property rights. The device is currently available in nearly 40 hospitals across China, enabling over 140 patients with advanced heart failure to embark on a “new life”.
Recently, at the 2022 China Medical Development Conference—widely regarded as the country’s premier medical forum—the Chinese Academy of Medical Sciences (CAMS) released two lists: China’s Major Medical Achievements of the 21st Century and China’s Important Medical Advancements in 2021. As a global innovator in the field of Ventricular Assist Devices (VADs), CH Biomedical’s independently developed CH-VAD (NMPA (A) 20213120987) was included in China’s Important Medical Advancements in 2021.
On November 24th 2021, the implantable CH-VAD® left ventricular assist system (CH-VAD® LVAS) of CH Biomedical was approved by the National Medical Products Administration (NMPA) for marketing (Registration No.: GXZZ 20213120987). CH-VAD LVAS was developed independently by CH Biomedical, it is the first Left Ventricular Assist Device (LVAD) with complete independent intellectual property rights approved by NMPA in China, and the first full magnetic levitation LVAD approved by NMPA.


