On May 11, 2024, the Fuwai Hospital single-center long-term outcomes of CH Biomedical’s CH-VAD, a fully magnetically levitated ventricular assist device were published online in The Journal of Heart and Lung Transplantation (JHLT), a leading international journal in cardiothoracic surgery, under the title “Long-term outcomes of a novel fully magnetically levitated ventricular assist device for the treatment of advanced heart failure in China” [1]. This marks the first instance of clinical research on an implantable ventricular assist device (VAD) from China being featured in this prestigious journal, as well as the inaugural appearance of research outcomes from an original medical device in China in the Journal for the first time.

JHLT, the official publication of the International Society for Heart and Lung Transplantation (ISHLT), focuses on high-quality original research, reviews, guidelines, and clinical trials in cardiothoracic surgery, mechanical circulatory support, pulmonary hypertension, and advanced heart and lung diseases. According to the latest Journal Citation Reports (JCR) from the Chinese Academy of Sciences, JHLT is rated as a top-tier (Q1) journal in the medical field, with an impact factor of 8.9 in 2022, highlighting its significant influence in cardiothoracic surgery.
This study, the largest single-center study on LVAD conducted in China to date, utilized CH-VAD, an independently developed fully magnetically-levitated LVAD from CH Biomedical. Led by Academician Hu Shengshou at Fuwai Hospital, the study was completed by a multidisciplinary team of leading domestic experts from cardiac surgery, cardiology, critical care medicine, ultrasound, anesthesiology, and nursing, including Wang Xianqiang, Zhou Xingtong, Chen Haibo, Du Juan, Qing Ping, Zou Liang, Chen Yi, Duan Fujian, Yuan Su, Shi Jia, Ji Bingyang, Wu Rong, Zhang Yanming, and Jin Ya.
Background of the Study
Heart failure is a major health concern globally. In China, approximately 1.3% of individuals over the age of 35 suffer from heart failure, with10-15% of these patients progressing to advanced stages, where prognosis significantly worsens. The application of LVAD has greatly improved the survival rates and quality of life for patients with advanced heart failure. Among various technological advancements, the fully magnetically levitated LVAD has become the most widely adopted worldwide due to the superior hemocompatibility. As the first and only fully magnetically levitated LVAD in China, CH-VAD has been in use since its initial application in 2017, benefiting nearly 300 patients with advanced heart failure to date.
To evaluate the safety and long-term efficacy of CH-VAD in treating advanced heart failure, Fuwai Hospital conducted this single-center, retrospective, observational study. The study reviewed 50 patients who received CH-VAD implantation between June 2017 and August 2023, reporting long-term follow-up outcomes.
Key Findings
•Innovative Technology: CH-VAD, the first and only fully magnetically levitated LVAD in China, features an innovative structural design that ensures excellent hemocompatibility and hemodynamic performance, safeguarding long-term, high-quality postoperative life for patients with advanced heart failure.
•Significant Efficacy: High survival rates and low complication rates demonstrate the safety and sustained effective long-term support of CH-VAD.
•International Recognition: The publication of the study marks a significant breakthrough in the academic field of LVADs in China, gaining international recognition for China’s innovation in medical technology.
Innovative Technology of CH-VAD
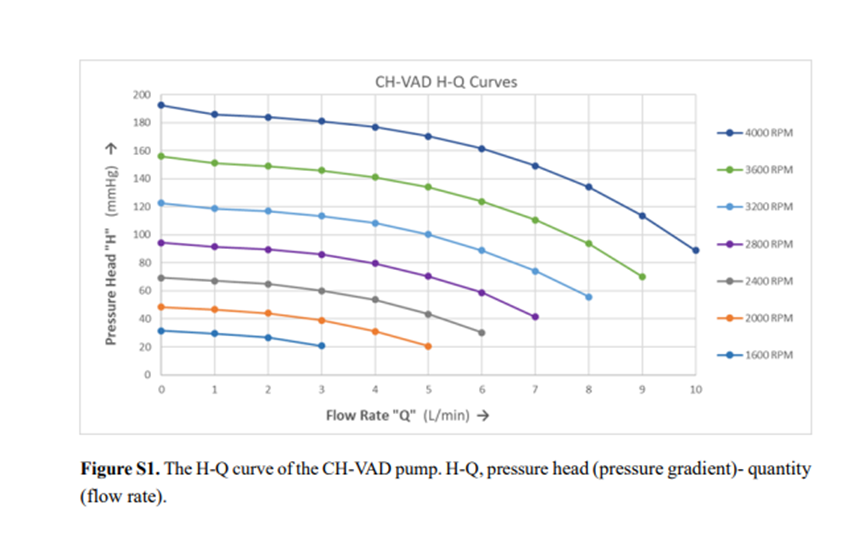
•World’s First Separated Fully Magnetically Levitated Structure: CH-VAD features a separated magnetic levitation and motor design, maximizing internal pump space utilization to achieve a compact pump size measuring 47 mm in diameter and 25 mm in thickness, with an expanded rotor diameter of 33 mm. The larger rotor diameter results in a flatter H-Q curve and lowers the pump’s operating speed, enhancing blood flow pulsatility. Operating at just 2800 RPM, the 33 mm rotor delivers a 5 L/min flow rate under a 70-mmHg pressure gradient, while the lower speed significantly reduces turbulence and blood damage.
•Multidisciplinary-Optimized Pump Flow Path Design: The flow path design is a critical factor in enhancing the hemocompatibility of the pump. As illustrated in Figure B, CH-VAD integrates both primary and secondary flow paths. The primary flow path features a Nose Cone, which smoothly redirects blood flow in the inflow cannula, preventing stagnation from direct impact at the inflow base. The secondary flow path, with a 0.25 mm gap width, has undergone extensive optimization to ensure adequate flushing, minimizing blood exposure to high shear stress, reducing turbulence, and preventing Taylor vortices, thereby achieving superior hemocompatibility.
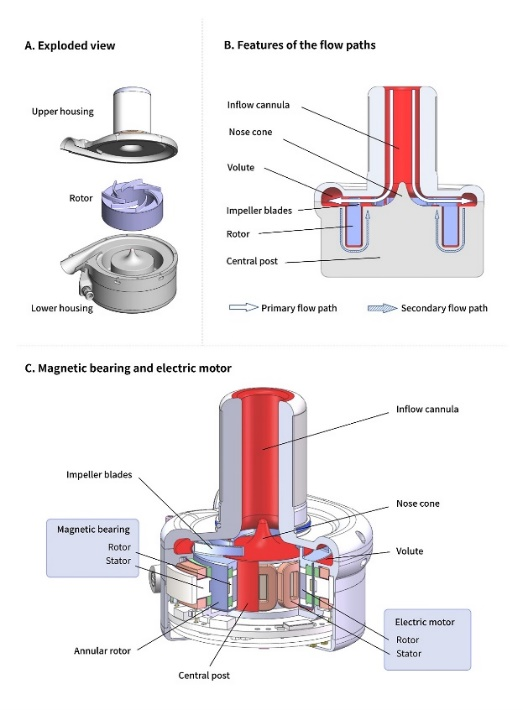
Design Schematic Diagram of the CH-VAD Pump
•Unique Transcutaneous Driveline Communication and Power Supply Technology: The driveline is designed to be thinner and more flexible, with an outer diameter of just 3.3 mm, significantly reducing the risk of driveline-related infections by design.
Significant Long-Term Clinical Efficacy
Baseline Characteristics:
The study included 50 patients with a mean age of 47.9 ± 13.9 years (range: 15-73) and an average body surface area (BSA) of 1.89 ± 0.18 m². Among them, 13 patients (26%) had ischemic heart disease as the underlying etiology. At baseline, all patients were classified as New York Heart Association (NYHA) functional Class IV. The INTERMACS profiles were as follows: 6 patients (12%) at Level 1, 28 patients (56%) at Level 2, 13 patients (26%) at Level 3, and 3 patients (6%) at Level 4. Additionally, 15 patients (30%) required temporary mechanical circulatory support prior to surgery. 68% of patients were classified as INTERMACS Levels 1-2, a proportion significantly higher than the 10-48% reported in international clinical trials and registries [2-3], indicating more severe conditions and heightened clinical challenges.
Clinical Outcomes:
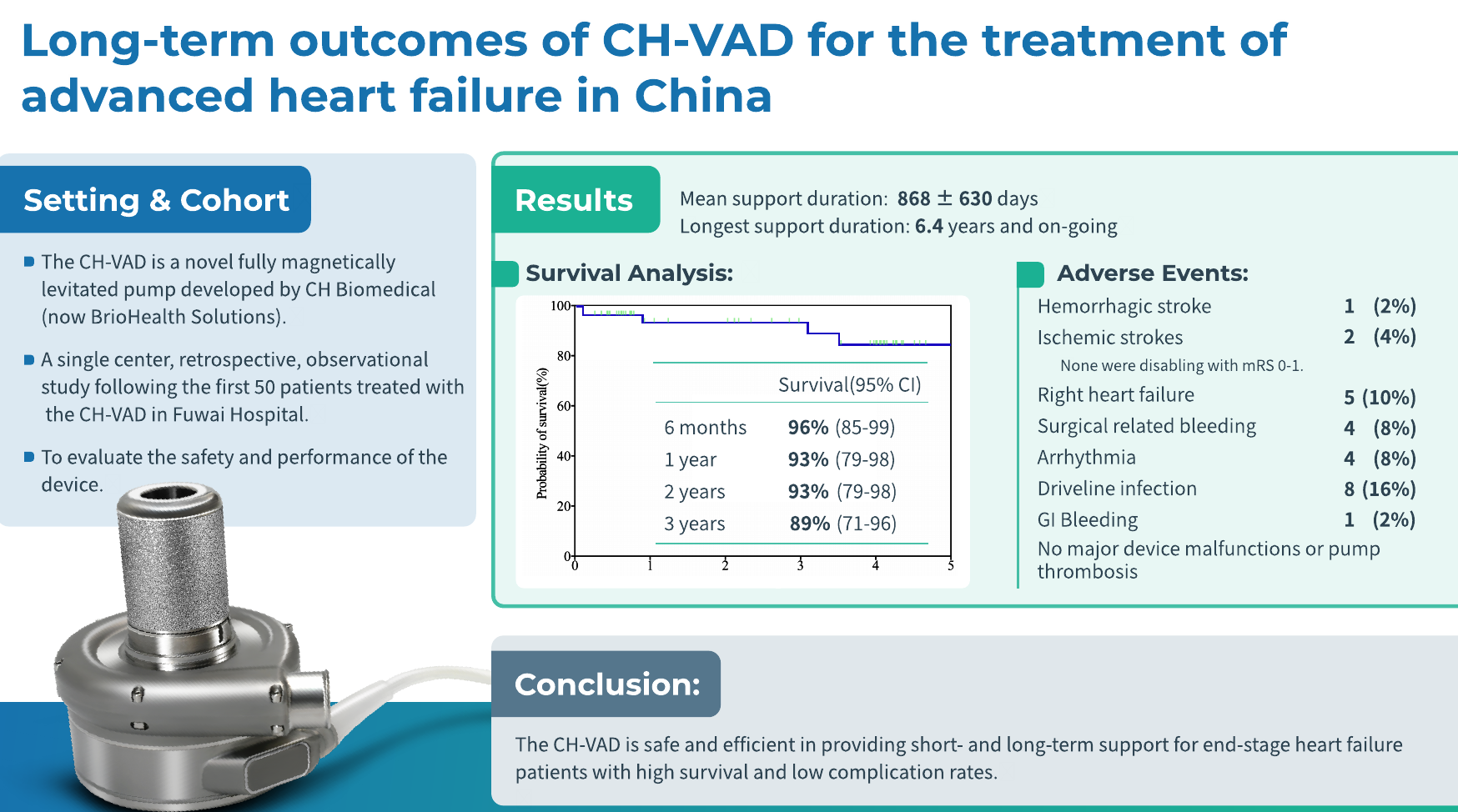
The mean duration of support was 868 ± 630 days (range: 33 days to 6.4 years). Survival rates were 96% at 6 months, 93% at 1 year, 93% at 2 years, and 89% at 3 years, indicating excellent long-term survival outcomes. By the end of the follow-up period, 40 patients (80%) remained alive with the pump in place, 3 patients experienced cardiac function recovery and had the pump removed, 2 patients transitioned to heart transplantation, and 5 patients died during support.
The 2023 INTERMACS Annual Report [2] presented long-term survival rates for the total magnetically levitated LVADs globally (see figure). Compared to international outcomes, the long-term survival rates achieved with CH-VAD at Fuwai Hospital were notably superior (1-year survival: 93% vs. 86%; 2-year survival: 93% vs. 79%).
Adverse Events:
Adverse events (AEs) in the study were defined based on INTERMACS criteria. The most frequent adverse events included percutaneous driveline infections (16%, 0.08 EPPY), right heart failure (10%, 0.06 EPPY), surgery-related bleeding (8%, 0.03 EPPY), and arrhythmias (8%, 0.05 EPPY).
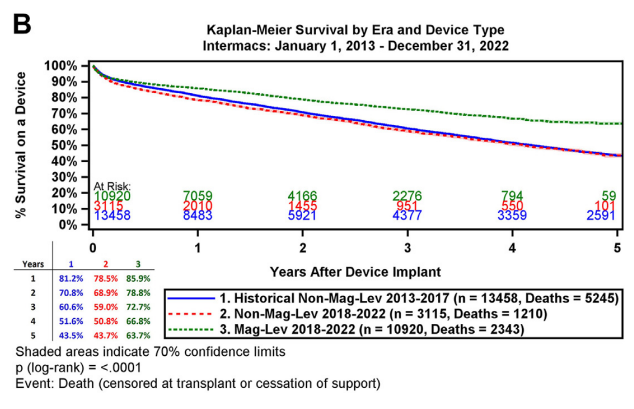
Since the MOMENTUM 3 trial, a landmark study in the history of LVAD development, demonstrated the advantages of the total magnetically levitated LVADs in enhancing long-term survival and reducing postoperative adverse events, the device has become the most widely adopted LVADs worldwide. The 2023 INTERMACS Annual Report [2] detailed the real-world clinical outcomes of the total magnetically levitated LVADs (Mag-Lev) over the past five years [2] (see the figure below). Compared to these international findings, the CH-VAD cohort at Fuwai Hospital showed lower rates of adverse events, including hemocompatibility-related complications and infections. This outcome can be attributed to stringent clinical quality control and the innovative design of CH-VAD.
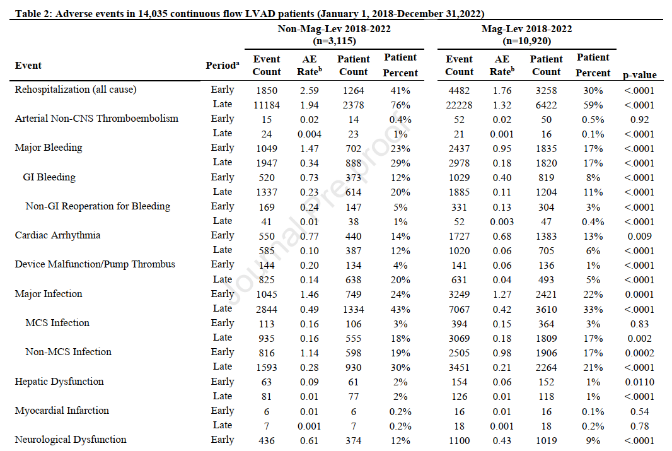
AEs reported in the INTERMACS Annual Report
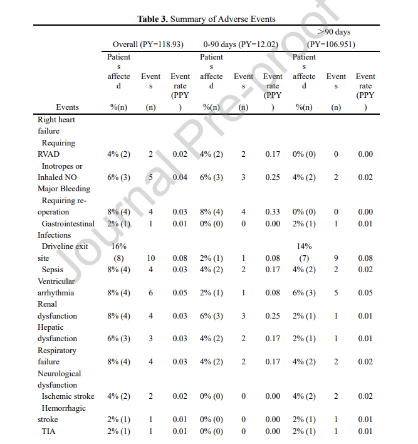
AEs observed in the single-center study at Fuwai Hospital
Low Incidence of Right Heart Failure:
Right Heart Failure (RHF) is a critical complication that impacts patient survival following LVAD implantation. Key factors influencing postoperative RHF include patient screening, preoperative optimization, and LVAD design play pivotal roles in influencing the occurrence of postoperative RHF.
The MOMENTUM 3 trial reported an RHF incidence of 34.2% [9] (see the figure below), with an EPPY of 0.82. In this study at Fuwai Hospital, RHF was observed in 5 out of 50 patients, corresponding to an incidence of 10% and an EPPY of 0.06, which is significantly lower than the MOMENTUM 3 data. Additionally, 4 out of the 5 RHF cases (80%) occurred during the early clinical trial or humanitarian assistance phase. With advancements in patient selection and preoperative optimization, RHF incidence was markedly reduced.
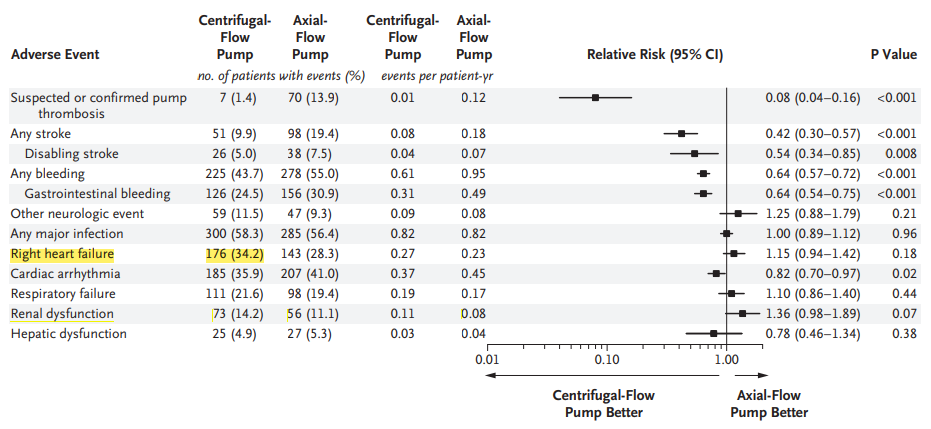
The CH-VAD features multiple design elements aimed at reducing the RHF risk. Its smaller size minimizes the likelihood of left ventricular apical compression and ventricular deformation. Additionally, the relatively flat H-Q curve of CH-VAD enhances the pump’s sensitivity to preload variations, effectively unloading the left ventricle while reducing the risk of suction. In practice, adjusting the pump speed allows for maintaining appropriate cardiac output while mitigating right ventricular anatomical changes, thereby significantly lowering the likelihood of RHF.
Low Incidence of Hemocompatibility-Related Adverse Events:
Hemocompatibility is a critical factor influencing the long-term clinical outcomes of LVADs. Complications related to hemocompatibility, such as pump thrombosis, stroke, and gastrointestinal bleeding, have a direct impact on postoperative survival rates and quality of life. The CH-VAD enhances hemocompatibility through a larger rotor size and an optimized flow path design. Findings from laboratory testing [4], hydrodynamic analysis [5], and preclinical animal studies [6-8] have been continuously published in journals such as Artificial Organs since 2015. In this study at Fuwai Hospital, no cases of pump thrombosis were reported, and the incidences of stroke and gastrointestinal bleeding were notably low. These findings provide robust clinical evidence supporting the superior hemocompatibility of CH-VAD.

Three patients achieved cardiac function recovery and underwent pump removal, while two patients transitioned to transplantation. Examination of the removed pumps in the operating room revealed clean flow paths with no evidence of thrombosis
Low Incidence of Postoperative Infections:
Infections are the most common adverse events following LVAD implantation. According to the INTERMACS 2023 report, the global device-related infection rates for LVADs are 0.15 EPPY within the first three months post-implantation and 0.18 EPPY thereafter. In this study, the incidence of postoperative driveline infections was significantly lower, with an EPPY of just 0.08. This reduction is attributed to the comprehensive and meticulous postoperative management by the Fuwai Hospital medical team, as well as the thinner and more flexible drivelines of CH-VAD, which reduce the likelihood of postoperative infections.
Currently, the only total magnetically levitated LVAD commercially available worldwide is Abbott’s HeartMate 3. Since its launch in the United States in 2017, HeartMate 3 has progressively replaced earlier LVAD models, including the HeartMate II, due to its superior hemocompatibility, which enhances patient survival and quality of life. Similarly, CH-VAD was firstly introduced in 2017, marking its clinical debut in China. The first patient implanted with CH-VAD has now survived for seven years, demonstrating its long-term clinical efficacy. The high-quality long-term outcomes of CH-VAD at Fuwai Hospital highlight the synergy of internationally leading clinical quality control measures and innovative product design. The publication of this study signifies international recognition of China’s advancements in LVAD technology and marks a major breakthrough in aligning China’s advanced heart failure treatment and LVAD applications with global standards.
References:
[1]. Long-term outcomes of a novel fully magnetically levitated ventricular assist device for the treatment of advanced heart failure in China
[2]. Intermacs 2023 annual report.
[3]. Fully Magnetically Levitated Left Ventricular Assist Device — Final Report
[4]. Berk ZBK, Zhang J, Chen Z, et al. Evaluation of in vitro hemolysis and platelet activation of a newly developed maglev LVAD and two clinically used LVADs with human blood. Artif Organs. 2019;43(9):870-9.
[5]. Zhang J, Chen Z, Griffith BP, et al. Computational characterization of flow and blood damage potential of the new maglev CH-VAD pump versus the HVAD and HeartMate II pumps. Int J Artif Organs. 2020;43(10):653-62.
[6]. Lin C, Wu G, Liu X, et al. In vivo evaluation of an implantable magnetic suspending left ventricular assist device. Int J Artif Organs. 2015;38(3):138-45.
[7]. Wang Y, Smith PA, Handy KM, et al. In vivo Hemodynamic Evaluation of an Implantable Left Ventricular Assist Device in a Long-term Anti-coagulation Regimen(). Annu Int Conf IEEE Eng Med Biol Soc. 2020;2020:2589-93.
[8]. Xu C, Wu G, Liu X, et al. Preclinical Study of Anticoagulation Regimens in Sheep After Implantation of CH-VAD Blood Pump. Artif Organs. 2018;42(9):891-8.
[9]. Mehra MR, Uriel N, Naka Y, et al. A Fully Magnetically Levitated Left Ventricular Assist Device - Final Report. N Engl J Med. 2019 Apr 25;380(17):1618-1627.
On August 7, People’s Daily published an article titled Domestic Fully Magnetically Levitated VAD Serving Patients, commending CH Biomedical for breaking the international technological monopoly for developing a domestically produced, fully magnetically levitated VAD with independent intellectual property rights. The device is currently available in nearly 40 hospitals across China, enabling over 140 patients with advanced heart failure to embark on a “new life”.
Recently, at the 2022 China Medical Development Conference—widely regarded as the country’s premier medical forum—the Chinese Academy of Medical Sciences (CAMS) released two lists: China’s Major Medical Achievements of the 21st Century and China’s Important Medical Advancements in 2021. As a global innovator in the field of Ventricular Assist Devices (VADs), CH Biomedical’s independently developed CH-VAD (NMPA (A) 20213120987) was included in China’s Important Medical Advancements in 2021.
On November 24th 2021, the implantable CH-VAD® left ventricular assist system (CH-VAD® LVAS) of CH Biomedical was approved by the National Medical Products Administration (NMPA) for marketing (Registration No.: GXZZ 20213120987). CH-VAD LVAS was developed independently by CH Biomedical, it is the first Left Ventricular Assist Device (LVAD) with complete independent intellectual property rights approved by NMPA in China, and the first full magnetic levitation LVAD approved by NMPA.


