From April 10 to 13, 2024 (local time), the 44th Annual Conference of the International Society for Heart and Lung Transplantation (ISHLT 2024) was held at the Prague Congress Centre in the Czech Republic. Renowned as a leading global academic event in the field of cardiopulmonary diseases, the ISHLT conference highlights cutting-edge academic topics such as heart and lung transplantation, advanced cardiopulmonary disease treatments, and mechanical circulatory support (MCS). By sharing the latest research and providing professional education, the event drives the global advancement of advanced cardiopulmonary disease treatment strategies and disciplines, enhancing outcomes for cardiopulmonary diseases.
On the afternoon of April 13 (local time), at the ISHLT session titled “Spin Doctors and Heart Hotties: Unleashing the Future of MCS Devices,” Professor Wang Xianqiang from Fuwai Hospital made the first-ever international presentation of the long-term follow-up results for CH-VAD. CH-VAD is the first fully magnetically levitated left ventricular assist device (LVAD) to become commercially available in China, featuring comprehensive independent intellectual property rights and several key technological breakthroughs. This single-center, retrospective, observational study demonstrated that patients with end-stage heart failure treated with CH-VAD achieved high survival rates and low complication rates, with no reported cases of pump thrombosis, disabling strokes or major device malfunctions. These groundbreaking findings demonstrated the outstanding potential of CH-VAD, garnering widespread acclaim from attending experts!

Professor Wang Xianqiang Delivered a Keynote Report at the Conference
Study Overview
In 2017, a team from Fuwai Hospital, led by Academician Hu Shengshou performed the China’s first CH-VAD implantation in a severe heart failure patient who was on ECMO and IABP support. This marked the beginning of the LVAD therapy for advanced heart failure in China. The patient remains alive nearly 7 years post-implant , enjoying a good quality of life and returning to normal work and daily activities.
Since the initial implantation in 2017, China’s LVAD field has advanced rapidly. Fuwai Hospital has developed comprehensive clinical protocols covering patient selection, surgical implantation, and postoperative management, and has assembled a multidisciplinary team with deep experience—becoming the country’s largest LVAD implantation center.
The CH-VAD device received regulatory approval in November 2021, supported by key evidence from clinical research led by Fuwai Hospital. To further assess the device’s long-term efficacy and safety, Fuwai Hospital conducted this single-center, retrospective, observational study, including all patients treated with CH-VAD from June 2017 to August 2023. Statistical analyses were performed on their clinical data and long-term outcomes.
The study enrolled 50 patients, with follow-up durations ranging from 3 months to 6.7 years. The average duration of pump support was 2.4 years, with a cumulative support duration of 119 patient-years. The average patient age was 48, with 90% male and an average body surface area of 1.89 m². The most common cause of heart failure was dilated cardiomyopathy (56%), followed by ischemic cardiomyopathy (26%) and valvular heart disease (12%). Fifteen patients (30%) required temporary mechanical circulatory support preoperatively, and 68% were classified as INTERMACS levels 1 and 2. All 50 patients successfully underwent LVAD implantation, with stable intra- and postoperative pump operation and hemodynamic recovery.
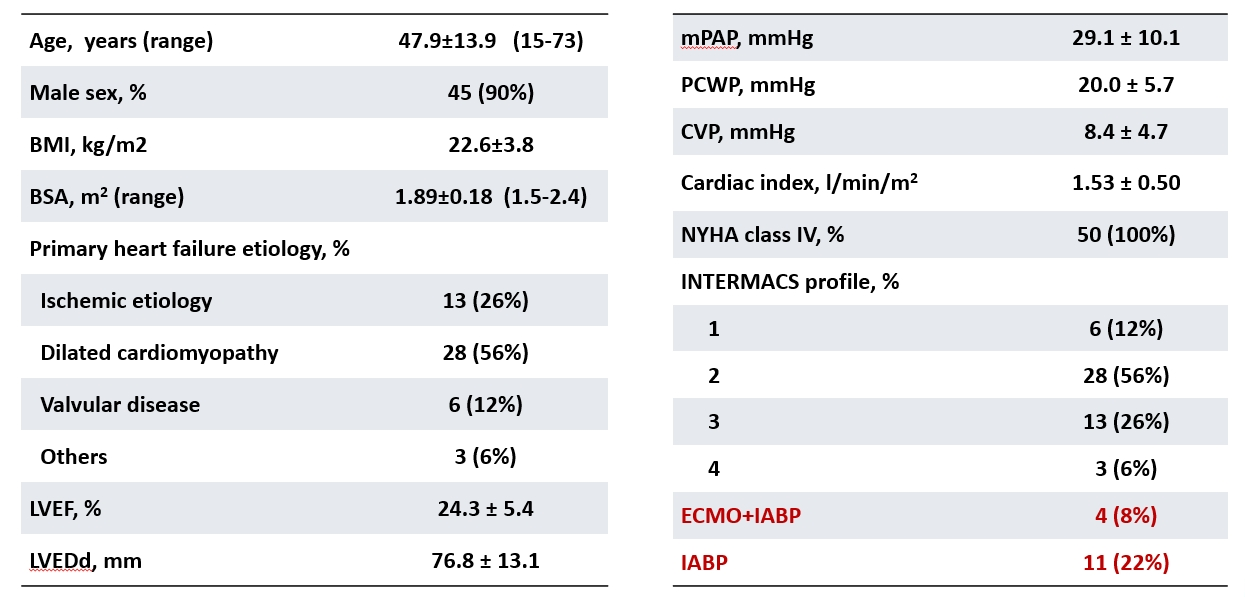
Table 1. Patient Baseline Data
Kaplan-Meier analysis revealed survival rates of 93% at 1 and 2 years, and 89% at 3 years (Figure 1), exceeding those reported in international clinical and real-world studies. Among the 50 patients, only 2 bridged to transplant, while 3 recovered cardiac function and underwent LVAD removal surgery (Figure 2).
.png)
Figure 1. Patient Survival Rates During Follow-Up
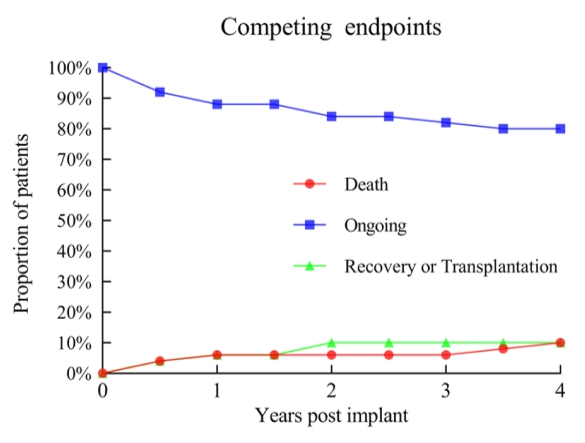
Figure 2. Incidence of Different Endpoint Events
The overall incidence of adverse events in this study was low. CH-VAD exhibited excellent hemocompatibility, with no observed cases of pump thrombosis, hemolysis, or disabling stroke. Among all patients, only two cases of ischemic stroke (0.02 events/patient-year) and one case of hemorrhagic stroke (0.01 events/patient-year) were observed, all with full neurological recovery during follow-up. Gastrointestinal bleeding occurred in only one patient, which was associated with excessively high INR levels.
Postoperative infection was the most common adverse event, but with growing multidisciplinary expertise, infection control improved significantly, leading to favorable patient outcomes. No major device malfunctions or pump replacement surgeries occurred, indicating the device’s high long-term reliability. (Table 2)
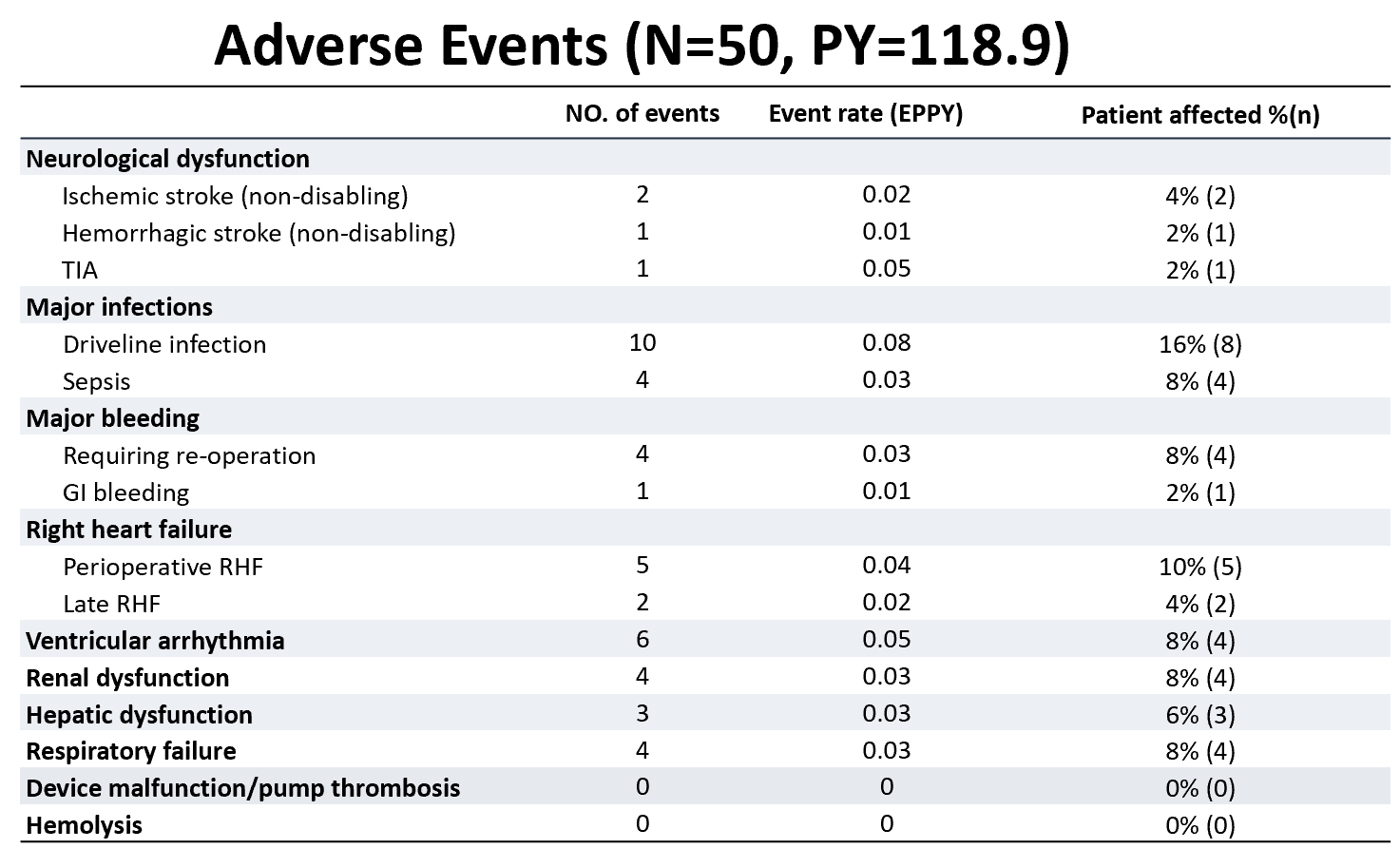
Table 2. Incidence of Various Adverse Events
The study also analyzed hospital readmission. The proportions of patients remaining free from readmission at 1, 2, and 3 years was 83%, 62%, and 53%, respectively—significantly higher than INTERMACS benchmarks, indicating that CH-VAD recipients maintain a good quality of life.
A total of 20 patients accounted for 31 unplanned readmissions, mainly due to severe infections, heart failure-related events, diagnostic evaluation, and neurological events. (Figure 3)
.png)
Figure 3. Proportion of Patients Free from Readmission During Follow-Up
The study demonstrated that CH-VAD provides excellent long-term survival rates with low complication and readmission rates. The incidence of hemocompatibility-related adverse events was extremely low, with no occurrences of pump thrombosis, hemolysis, or disabling strokes. As the first fully magnetically levitated LVAD approved for clinical use in China, CH-VAD integrates multiple innovative design features. Its outstanding performance has enabled patients to achieve high-quality long-term survival and has been widely adopted for the treatment of heart failure in China. Statistics show that CH-VAD has been successfully implanted in over 220 patients at more than 50 clinical centers.
Cognition, Innovation, and Standardization: LVADs Empowering Heart Failure Patients to Achieve Long-Term High Quality of Life
LVAD therapy aims to provide high-quality, long-term survival for patients with advanced heart failure. The key to maximizing patient benefit is to act within the “golden window” for treatment—prior to irreversible multi-organ failure—rather than relegating LVAD as a last-resort option. Therefore, clinicians must promptly identify patients with advanced heart failure and proactively assess the potential benefits of LVAD therapy.
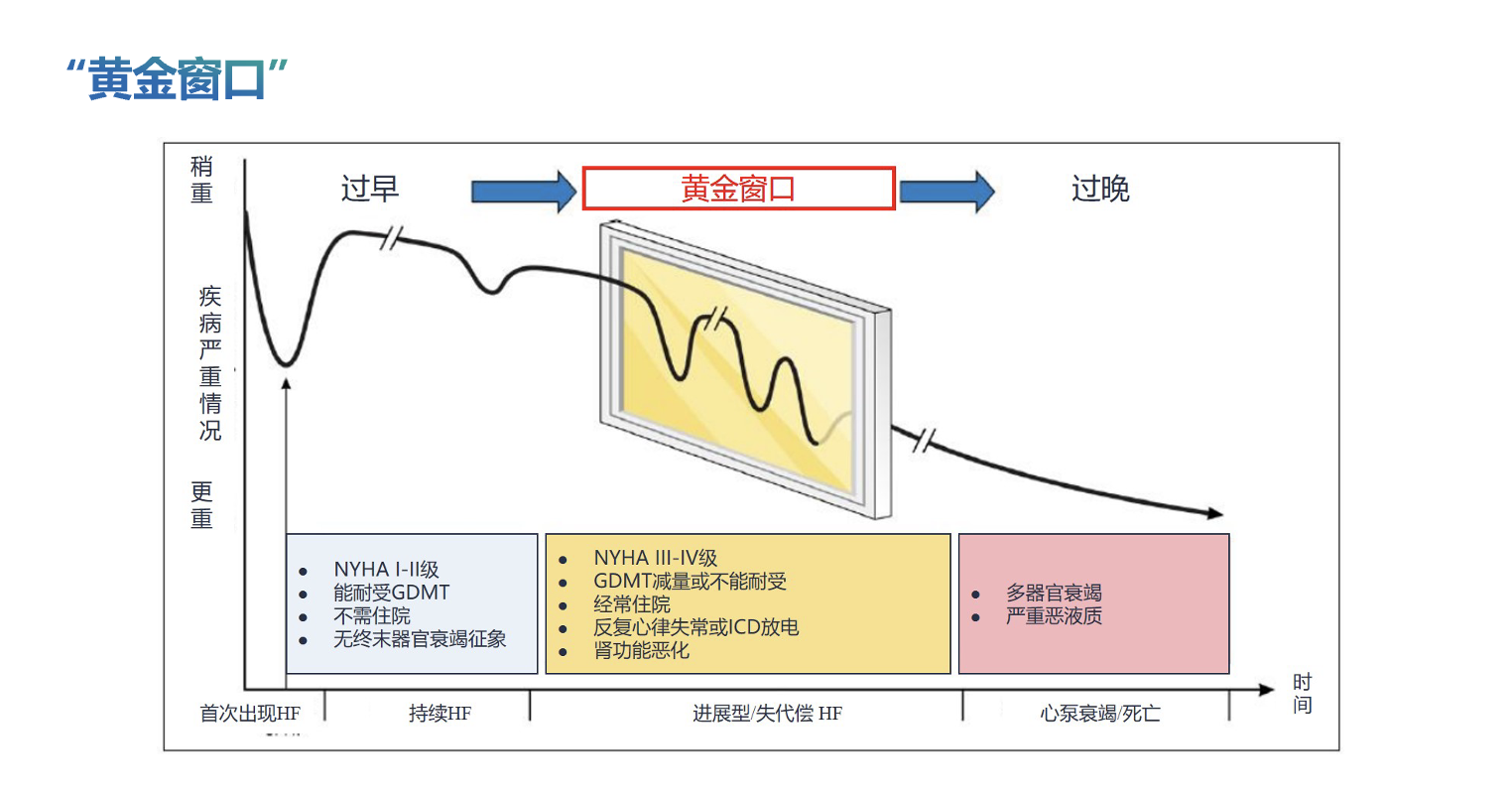
Figure 4. The Golden Window for Treatment
Selecting the right device is another critical factor in the success of LVAD therapy. Over two decades of exploration and technological accumulation, the clinical value of fully magnetically levitated devices in improving patient outcomes has gained widespread recognition within the medical community, establishing them as the mainstream technology for LVADs. CH-VAD has further achieved multiple technological breakthroughs via multidisciplinary optimization, particularly in magnetic levitation structure and flow path design. These innovations enhance hemocompatibility, contributing to superior clinical outcomes. Additionally, the device benefits from a comprehensive global patent portfolio.
CH-VAD features an innovative segmented fully magnetically levitated structure that optimizes pump space efficiency, allowing a large impeller to be housed within a compact pump body. Its 33mm-diameter impeller utilizes a cantilevered long blade design, significantly reducing rotational speed while maintaining the same flow rate. This design lowers shear stress between the blades and the suspension gap, minimizing blood damage. Furthermore, the segmented design achieves suspension stiffness capable of withstanding accelerations up to 6g, ensuring stable magnetic suspension of the impeller during high-speed rotation. Even under varying physiological conditions and external forces, this design prevents impeller-to-casing contact, effectively reducing potential blood damage.
CH-VAD’s flow path design further enhances hemocompatibility. Blood entering the pump body is smoothly redirected via the nose cone guide, avoiding stagnation or turbulence caused by direct impact at the inlet base. Blood entering the secondary flow path is vertically redirected into the main flow path through a U-shaped conduit, with an optimized angle and width that ensures uniform blood distribution and thorough flushing. This minimizes blood stasis and turbulence, reducing the risks of hemolysis and thrombosis. These integrated design features establish CH-VAD’s superior hemocompatibility, providing reliable long-term support for optimal post-implantation clinical outcomes.
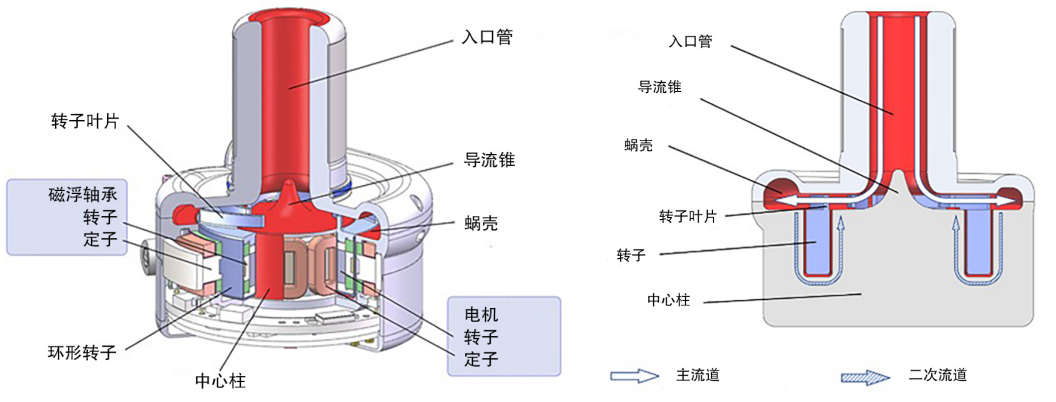
Figure 5. CH-VAD Blood Pump Design
Comprehensive and meticulous whole-process management is essential for ensuring long-term postoperative benefits for patients. From patient selection, preoperative optimization, surgical implantation, perioperative management, to long-term follow-up, every step requires the multidisciplinary team to execute their roles effectively and work in close coordination. Standardized management is crucial for facilitating patients' rapid recovery and reintegration into society.
The success of LVAD therapy is built on three key pillars: right patient, right device, and right management. As the first presentation of CH-VAD’s long-term efficacy in the international arena, Fuwai Hospital’s single-center clinical experience has demonstrated the device’s safety, efficacy and reliability. With growing global clinical validation, CH-VAD holds promise as an ideal solution for advanced heart failure patients worldwide, empowering them to embrace a better, brighter life.
On August 7, People’s Daily published an article titled Domestic Fully Magnetically Levitated VAD Serving Patients, commending CH Biomedical for breaking the international technological monopoly for developing a domestically produced, fully magnetically levitated VAD with independent intellectual property rights. The device is currently available in nearly 40 hospitals across China, enabling over 140 patients with advanced heart failure to embark on a “new life”.
Recently, at the 2022 China Medical Development Conference—widely regarded as the country’s premier medical forum—the Chinese Academy of Medical Sciences (CAMS) released two lists: China’s Major Medical Achievements of the 21st Century and China’s Important Medical Advancements in 2021. As a global innovator in the field of Ventricular Assist Devices (VADs), CH Biomedical’s independently developed CH-VAD (NMPA (A) 20213120987) was included in China’s Important Medical Advancements in 2021.
On November 24th 2021, the implantable CH-VAD® left ventricular assist system (CH-VAD® LVAS) of CH Biomedical was approved by the National Medical Products Administration (NMPA) for marketing (Registration No.: GXZZ 20213120987). CH-VAD LVAS was developed independently by CH Biomedical, it is the first Left Ventricular Assist Device (LVAD) with complete independent intellectual property rights approved by NMPA in China, and the first full magnetic levitation LVAD approved by NMPA.


