
May 24-27, 2022 International Society for Mechanical Circulatory Society (ISMCS) and European Mechanical Circulatory Society (EUMS) Joint Scientific Conference was successfully held in Hanover, Germany. Dr. Frank Lin, PhD, CTO of CH Biomedical, and Mr. Karl Nelson, Vice President of Clinical Operations were invited to attend the conference. Dr. Lin delivered a plenary speech at the VAD UPDATES sub-session and shared the latest progress and relevant data on the clinical use of its first LVAD in China. This has been the fourth time for the company to attend the influential conference hosted by ISMCS since 2018, and the company's self-developed totally magnetically levitated LVAD has been highly recognized among the international community.
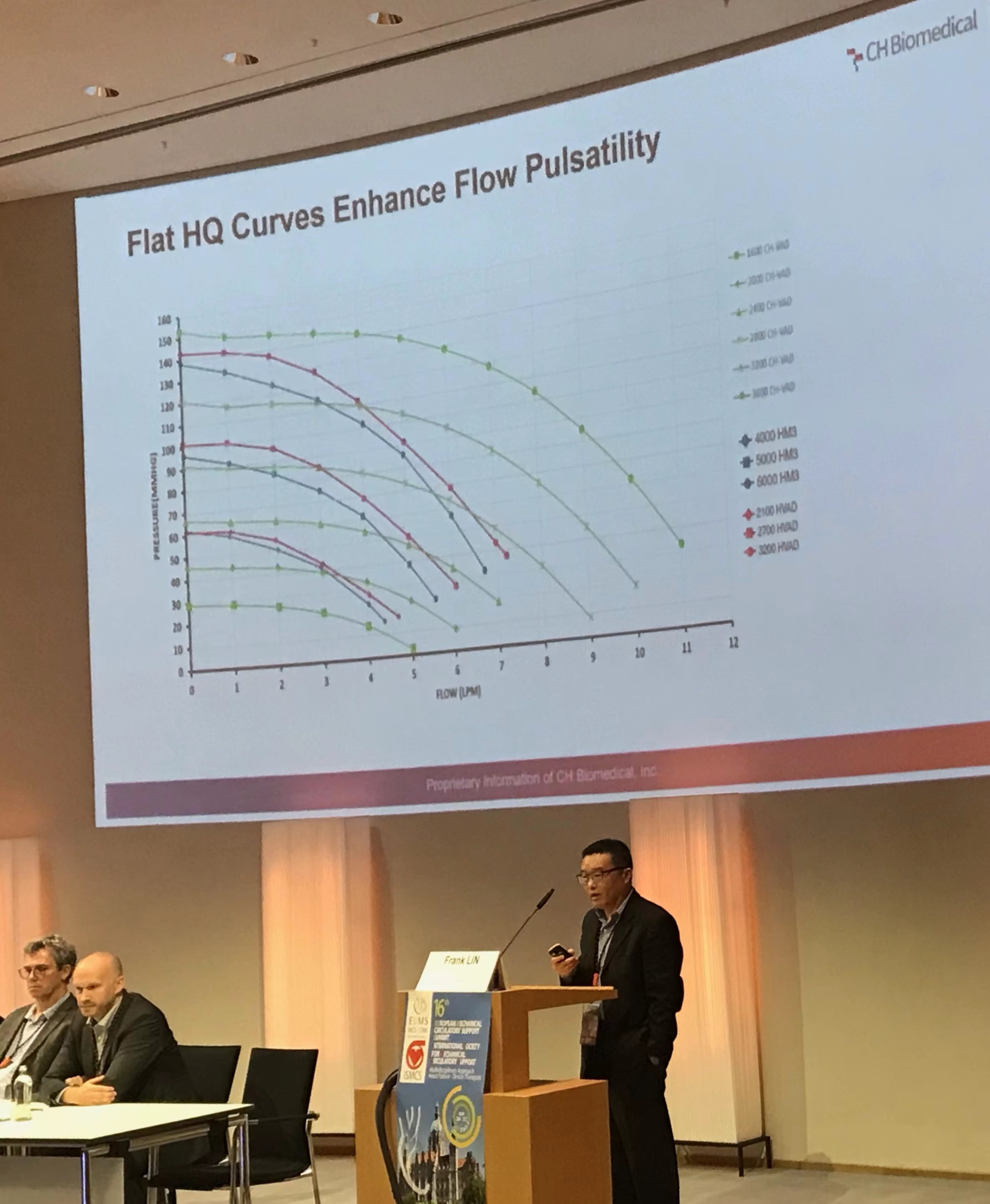
At the conference, Dr. Lin not only announced the exciting news that the LVAD has been officially approved in China, but also highlighted the outstanding technical advantages and features of the product and the excellent clinical trial results in China. It is also the first totally magnetically levitated LVAD approved by China’s NMPA, which has demonstrated great potentials in key performance indicators such as hemocompatibility, implant invasion, and reliability; no serious adverse events related to hemocompatibility were found such as pump thrombosis, stroke, or GI bleeding among all clinical use cases so far.
Dr. Lin also stated at the conference that the company is accelerating the process and planning to expand global access to its lifesaving technology and next-generation maglev LVAD in the United States and Europe.
The speech also generated strong interest and great response among top clinicians and experts at the conference who expressed keen interest in this innovative technology that might help further reduce the possibilities of adverse events after LVAD implantation.

Dr. Aly Banayosy, ISMCS President said the LVAD space desperately needs a new product and the timing couldn’t be better for CH Biomedical to enter the market.

Dr. Jan Schmitto, President of EUMS, leading cardiac surgeon at Hannover Medical School expressed strong desire to help the company design the clinical trial in Europe.
As one of the event organizer, ISMCS (International Society for Mechanical Circulatory Support) is dedicated to provide a broad international forum for intensive discussion of research, development, clinical use and social acceptance of rotary blood pumps and all related forms of mechanical circulatory support. CH Biomedical has been reporting on the progress of its LVAD product development at important conferences hosted or co-hosted by ISMCS annually since 2019, and has had a number of research findings published in Artificial Organs, the official Journal of both the International Federation of Artificial Organs and ISMCS, which have generated a strong response from the international academic community.
In addition, the company is also active in a number of other top academic organizations around the world. The company has been invited to the annual meeting of the American Society for Artificial Internal Organs (ASAIO) for more than ten years, and in 2019 reported on the progress of the Chinese clinical trial of its first LVAD product, which attracted strong attention. The Gordon Research Conference has been inviting the company to participate its annual networking event since 2009.
On August 7, People’s Daily published an article titled Domestic Fully Magnetically Levitated VAD Serving Patients, commending CH Biomedical for breaking the international technological monopoly for developing a domestically produced, fully magnetically levitated VAD with independent intellectual property rights. The device is currently available in nearly 40 hospitals across China, enabling over 140 patients with advanced heart failure to embark on a “new life”.
Recently, at the 2022 China Medical Development Conference—widely regarded as the country’s premier medical forum—the Chinese Academy of Medical Sciences (CAMS) released two lists: China’s Major Medical Achievements of the 21st Century and China’s Important Medical Advancements in 2021. As a global innovator in the field of Ventricular Assist Devices (VADs), CH Biomedical’s independently developed CH-VAD (NMPA (A) 20213120987) was included in China’s Important Medical Advancements in 2021.
On November 24th 2021, the implantable CH-VAD® left ventricular assist system (CH-VAD® LVAS) of CH Biomedical was approved by the National Medical Products Administration (NMPA) for marketing (Registration No.: GXZZ 20213120987). CH-VAD LVAS was developed independently by CH Biomedical, it is the first Left Ventricular Assist Device (LVAD) with complete independent intellectual property rights approved by NMPA in China, and the first full magnetic levitation LVAD approved by NMPA.


