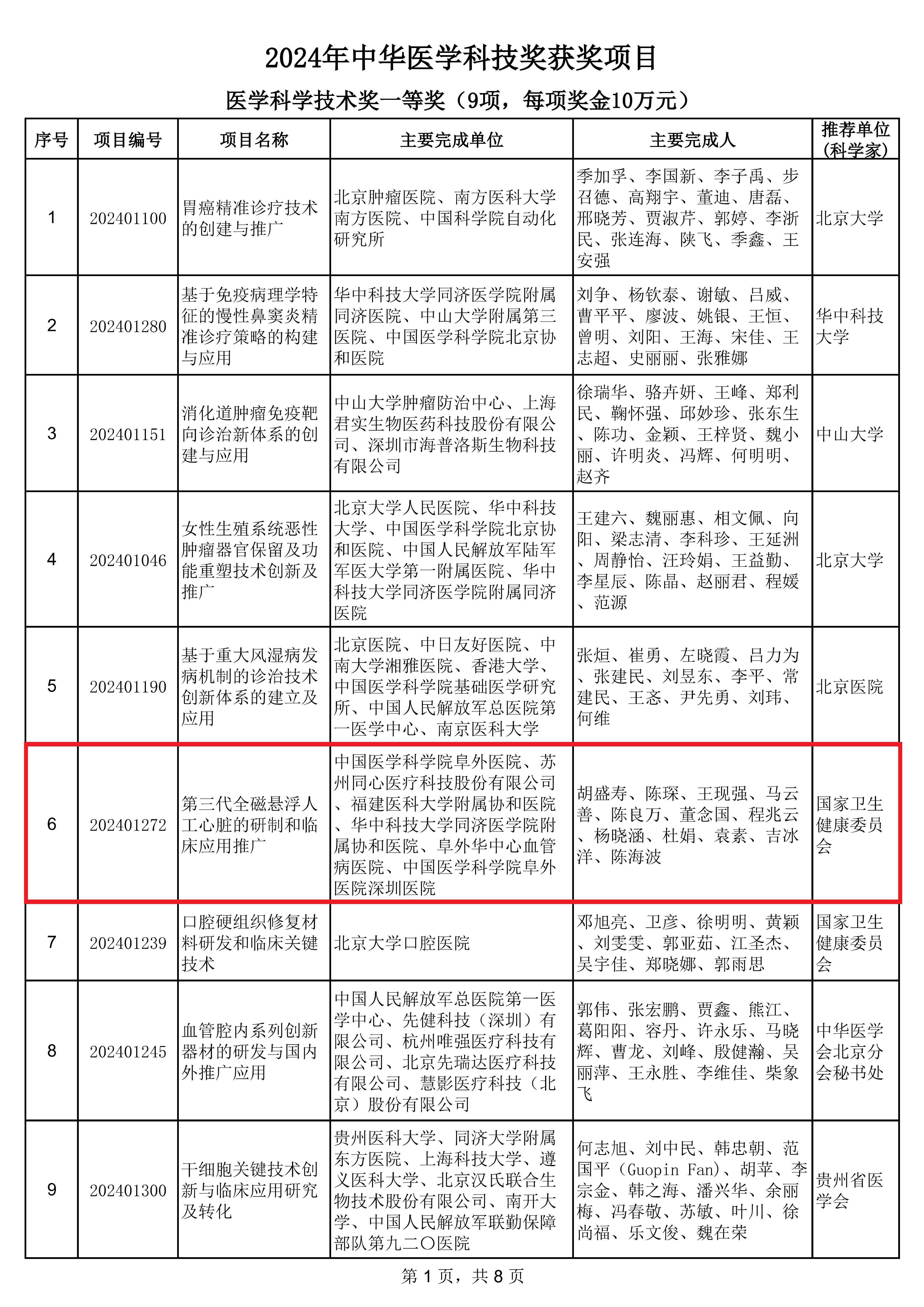
Recently, the project titled “Development and Clinical Application of the Third-Generation Fully Magnetically Levitated Left Ventricular Assist Device (LVAD),” led by Academician HU Shengshou, Director of the National Center for Cardiovascular Diseases and President of Fuwai Hospital (CAMS & PUMC), and jointly completed by BrioHealth Technologies and multiple leading clinical centers in China, was awarded the First Prize at the 2024 Chinese Medical Science and Technology Awards by the Chinese Medical Association (CMA), with BrioHealth Technologies recognized as the second leading contributor. The project was recommended by the National Health Commission, reviewed by experts from the CMA’s Award Review Committee, and formally approved at the 21st Executive Council Meeting of the 26th CMA Board of Directors.
The Chinese Medical Science and Technology Award is a prestigious national honor established by the CMA to recognize outstanding individuals and teams in the medical and health sector. It aims to promote scientific and technological advancement and innovation in the field of medicine in China. The award covers a wide range of disciplines including natural sciences, technological inventions, and scientific and technological progress in the pharmaceutical and medical fields. Held annually, it is considered one of the most authoritative and influential accolades in China’s healthcare industry.
Heart failure (HF) is a global medical challenge, often referred to as the “cancer of heart disease.” In China, over 10 million people suffer from HF, placing a substantial burden on the public healthcare system. While pharmacological treatments have advanced significantly, options remain limited for patients with advanced heart failure. Heart transplantation is severely limited by donor shortages. In contrast, VADs have become a globally recognized treatment for advanced, refractory HF. However, the development of VADs remains a major technical challenge worldwide. The most cutting-edge fully magnetically levitated LVAD technology is currently monopolized by international companies, and China had long lacked domestic breakthroughs in this field.
In response to this urgent medical need, under the leadership of Academician Hu, a team from Fuwai Hospital, in collaboration with BrioHealth Technologies and other top clinical experts across the country, tackled multiple core issues in the development and application of VAD technologies. The CH-VAD, a fully magnetically levitated LVAD with complete independent intellectual property rights, was successfully developed. Meanwhile, the team established comprehensive manufacturing techniques and a robust quality system.Through a deeply integrated medicine-engineering approach, the project achieved major breakthroughs: preclinical evaluations, human safety and efficacy studies, and surgical technique optimization. It developed standardized surgical procedures and multidisciplinary, full-cycle treatment protocols adaptable to different medical environments. These efforts validated the internationally advanced technical performance and clinical efficacy of CH-VAD, and enabled large-scale clinical adoption and commercialization in China.
This landmark scientific achievement has not only filled a major gap in China’s research, manufacturing, and clinical application of VADs, but also marked China’s transition from a technology follower to a global leader in the field through original innovation.

CH-VAD is the first in the world to achieve several key technological breakthroughs in the VAD field, including miniaturized magnetic levitation structures, optimized fluid dynamics design, and transcutaneous power cable technology. The system is backed by a comprehensive patent portfolio across China, the U.S., Europe, Japan, and other regions.Its performance in implant invasiveness, hemocompatibility, hemodynamics, and infection prevention meets or exceeds international benchmarks. Clinically, CH-VAD has demonstrated superior perioperative survival and long-term outcomes compared to international averages. Related innovations and results have been published in leading academic journals and presented at top-tier conferences, earning BrioHealth Technologies growing international recognition.
The project has achieved several historic firsts in China’s VAD field.In June 2017, under Academician Hu’s leadership, the first human implantation of CH-VAD as a novel clinical technology was completed—marking the beginning of durable VAD application in China.In November 2021, CH-VAD became the first domestically developed, fully magnetically levitated LVAD to receive market approval in China, ushering in the country’s “LVAD era.”In April 2024, BrioVAD, the next-generation device developed based on CH-VAD, became China’s first and only active implantable medical device to receive FDA approval for clinical trials in the United States. In November 2024, BrioVAD completed its first human implantation in the U.S., setting a new milestone in global HF treatment and marking China’s VAD debut on the international stage.
The success of this project brings new hope to countless HF patients and significantly enhances the country’s emergency and critical care capabilities. As a model of medicine-engineering collaboration, it reflects China’s national strategy of “innovation for the health of the people,” and serves as a landmark example of the industrialization of high-end medical devices in China.
On August 7, People’s Daily published an article titled Domestic Fully Magnetically Levitated VAD Serving Patients, commending CH Biomedical for breaking the international technological monopoly for developing a domestically produced, fully magnetically levitated VAD with independent intellectual property rights. The device is currently available in nearly 40 hospitals across China, enabling over 140 patients with advanced heart failure to embark on a “new life”.
Recently, at the 2022 China Medical Development Conference—widely regarded as the country’s premier medical forum—the Chinese Academy of Medical Sciences (CAMS) released two lists: China’s Major Medical Achievements of the 21st Century and China’s Important Medical Advancements in 2021. As a global innovator in the field of Ventricular Assist Devices (VADs), CH Biomedical’s independently developed CH-VAD (NMPA (A) 20213120987) was included in China’s Important Medical Advancements in 2021.
On November 24th 2021, the implantable CH-VAD® left ventricular assist system (CH-VAD® LVAS) of CH Biomedical was approved by the National Medical Products Administration (NMPA) for marketing (Registration No.: GXZZ 20213120987). CH-VAD LVAS was developed independently by CH Biomedical, it is the first Left Ventricular Assist Device (LVAD) with complete independent intellectual property rights approved by NMPA in China, and the first full magnetic levitation LVAD approved by NMPA.


