On November 25, 2024, BrioHealth Solutions, Inc., a wholly-owned U.S. subsidiary of BrioHealth Technologies, announced the completion of the first patient enrollment in the INNOVATE Trial of BrioVAD, a fully magnetically levitated left ventricular assist device (LVAD) developed by BrioHealth Technologies. The milestone was achieved at Emory University Hospital in the United States.

“We are thrilled to kick off the INNOVATE clinical trial. following a phenomenal journey of innovation, engineering, and quality refinement to bring the BrioVAD System to life,” said Dr. Chen Chen, Founder of BrioHealth Technologies and Chief Executive Officer of BrioHealth Solutions. “Despite advancements in ventricular assist devices, there remains a pressing need for improved device performance and patient outcomes, and BrioHealth is committed to addressing this gap. It is also incredibly rewarding to see the enthusiasm from our participating centers in advancing heart failure treatment through this study.”
Founded in 2008, BrioHealth Technologies has spent over a decade pioneering groundbreaking innovations, culminating in the development the CH-VAD, which is the first ultracompact, fully magnetically levitated LVAD with proprietary intellectual property approved by China’s National Medical Products Administration (NMPA). To date, more than 350 patients in China have been treated with CH-VAD, demonstrating outstanding clinical outcomes. BrioVAD has the same implantable pump as CH-VAD. Through multiple technological innovations, BrioVAD has achieved portability of the invitro components and enhanced overall system performance.
The INNOVATE trial is a prospective, non-blinded, randomized, controlled, multi-center, non-inferiority study designed to evaluate the safety and efficacy of the BrioVAD system in treating advanced, refractory left ventricular heart failure.

“Patients with advanced heart failure have limited options when it comes to treatment, with currently just one LVAD system available in the U.S., ”Francis D. Pagani, M.D., Ph.D., the Otto Gago, M.D., Professor of Cardiac Surgery at the University of Michigan Medical School and the study’s National Principal Investigator. “Despite treatment advances, complications still occur among LVAD patients. The INNOVATE Trial will provide important insights on if the BrioVAD System can help reduce complications and improve quality of life for patients with advanced heart failure.”

“The initiation of the INNOVATE Trial is an important milestone in advancing treatment options for advanced heart failure patients,” said Mani Daneshmand, M.D., Andrew J. McKelvey Professor, Emory University School of Medicine and Director, Emory Heart & Lung Transplantation and Mechanical Circulatory Support program. “We are excited to learn how the BrioVAD System can make an impact in this patient population.”
The initiation of the INNOVATE trial marks a significant step forward in global heart failure treatment and underscores BrioHealth Technologies’ commitment to its global strategy.
About the BrioVAD System
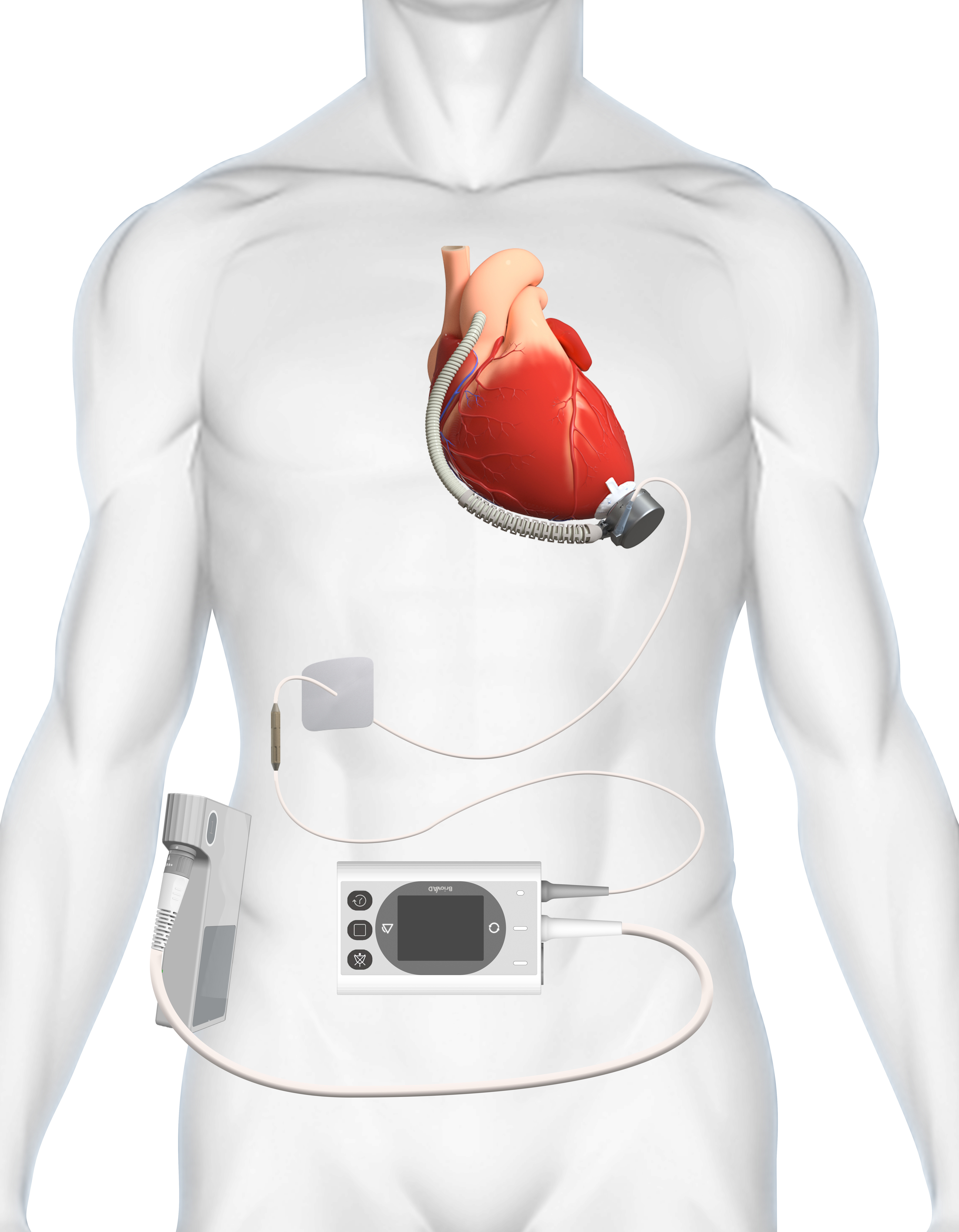

Main Components of the BrioVAD Left Ventricular Assist Device System
The BrioVAD system is a full-support, durable ventricular assist device comprised of an implantable BrioVAD pump and external components, designed to provide long-term support for heart failure patients. Compared to the only currently FDA-approved durable VAD in the U.S., the BrioVAD pump features an innovative magnetic bearing design that achieves a smaller pump size with a larger impeller, which may potentially reduce surgical invasiveness. Additionally, the BrioVAD pump incorporates a novel driveline design that electrically connects the pump to the external components, resulting in a significantly thinner and more flexible driveline, which may potentially reduce driveline-associated infections.
The combination of the BrioVAD Pump’s magnetic bearing construction and the large diameter impeller facilitates significant innovation in the design of the pump’s blood flow pathway within the pump. This advanced flow path design has the potential to improve device hemocompatibility and hemodynamics, minimizing the risk of serious complications.
The external components of the BrioVAD system incorporate several unique design features, requiring only two wearable units for daily use, significantly improving user experience and patient quality of life.
About the INNOVATE Trial

The INNOVATE Trial is a prospective, randomized, non-blinded, multi-center, confirmatory clinical trial that will enroll patients for both short-term and long-term mechanical circulatory support indications. The goal of the trial is to evaluate the safety and efficacy of the BrioVAD system in treating advanced, refractory heart failure. Participants will be randomized 2:1 to receive either BrioVAD or HeartMate 3. The primary endpoints of the trial include short-term (6 months) and long-term (24 months) survival rates without disabling stroke and pump replacement. Secondary endpoints include adverse events, cardiac function, quality of life and the total hospital days during follow-up.
Approximately 800 patients will be enrolled at leading medical centers across the U.S. For more information, visit http://www.theinnovatetrial.com.
On August 7, People’s Daily published an article titled Domestic Fully Magnetically Levitated VAD Serving Patients, commending CH Biomedical for breaking the international technological monopoly for developing a domestically produced, fully magnetically levitated VAD with independent intellectual property rights. The device is currently available in nearly 40 hospitals across China, enabling over 140 patients with advanced heart failure to embark on a “new life”.
Recently, at the 2022 China Medical Development Conference—widely regarded as the country’s premier medical forum—the Chinese Academy of Medical Sciences (CAMS) released two lists: China’s Major Medical Achievements of the 21st Century and China’s Important Medical Advancements in 2021. As a global innovator in the field of Ventricular Assist Devices (VADs), CH Biomedical’s independently developed CH-VAD (NMPA (A) 20213120987) was included in China’s Important Medical Advancements in 2021.
On November 24th 2021, the implantable CH-VAD® left ventricular assist system (CH-VAD® LVAS) of CH Biomedical was approved by the National Medical Products Administration (NMPA) for marketing (Registration No.: GXZZ 20213120987). CH-VAD LVAS was developed independently by CH Biomedical, it is the first Left Ventricular Assist Device (LVAD) with complete independent intellectual property rights approved by NMPA in China, and the first full magnetic levitation LVAD approved by NMPA.


