From November 13 to 15, 2024, the 30th International Society for Mechanical Circulatory Support (ISMCS 2024) conference was successfully held in Utsunomiya, Japan. As one of the prestigious high-level events in the field of mechanical circulatory support (MCS), the conference attracted clinicians, engineers, and experts from around the world to exchange cutting-edge research findings and practical experience. BrioHealth Technologies was invited to participate in this international academic gathering for the eighth consecutive year, where the company presented multiple research findings on its core products, the fully magnetically levitated left ventricular assist device, CH-VAD and BrioVAD. The presentations garnered widespread attention and high praise from the international academic community.
At the conference, Dr. Chen Chen, Founder of BrioHealth Technologies, provided an update on the research progress of CH-VAD and BrioVAD. Compared to the international counterparts, these devices demonstrate comprehensive performance advantages due to multidisciplinary design optimizations in areas such as implant invasion, hemocompatibility, hemodynamics, and infection prevention. The design benefits of these products are being validated in large-scale clinical applications in China. According to the latest follow-up data from a full cohort of CH-VAD clinical applications in China, the long-term survival rate of CH-VAD surpasses those in international large-scale clinical studies and INTERMACS data. Additionally, the incidence of severe adverse events, such as stroke, gastrointestinal bleeding, and percutaneous cable infections, is lower than those in international large-scale studies and INTERMACS data, consistently demonstrating world-leading clinical outcomes.
.jpg)
At the conference, Professor Wang Xianqiang from Fuwai Hospital, Chinese Academy of Medical Sciences, presented the long-term follow-up results for a full cohort of patients treated with CH-VAD at the hospital.
.jpg)
Patient Cohort: 88 patients were included, with follow-up durations ranging from 1.5 months to 7 years. On average, patients received pump support for 1.9 years, with a cumulative support time of 173 person-years.
Survival Rates: Kaplan-Meier analysis showed survival rates of 95% at 1 year, 95% at 2 years, and 91% at 3 years, exceeding those in international clinical studies and real-world data.
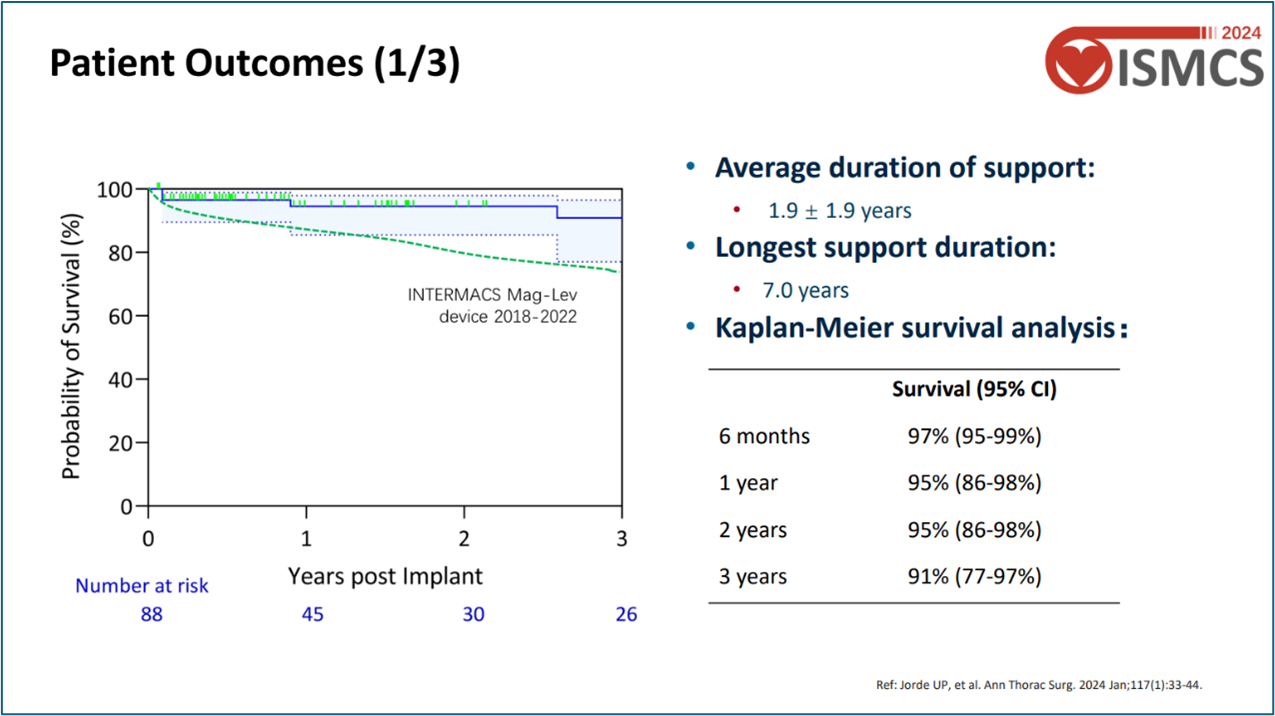
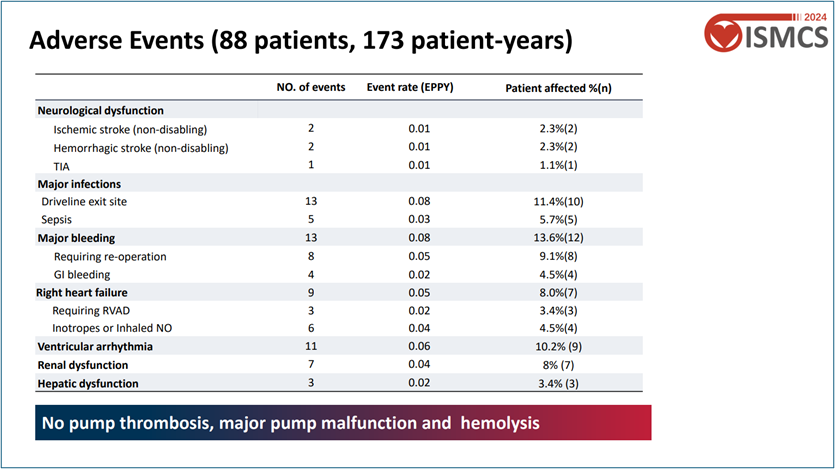
Device Reliability: No major device malfunctions, pump replacement surgeries, or hemolysis events were reported, indicating excellent long-term reliability.
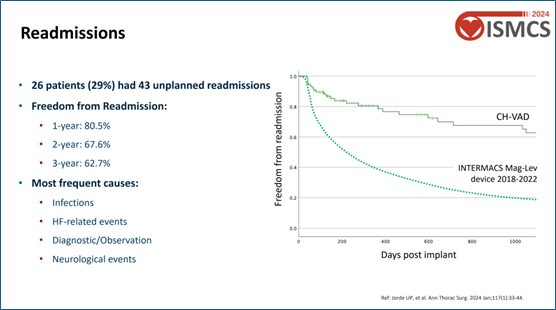
Readmission Statistics: The percentage of patients avoiding readmission was 80.5% at 1 year, 67.6% at 2 years, and 62.7% at 3 years --- significantly higher than the INTERMACS data, highlighting enhanced quality of life.
Professor Gong Ming from Beijing Anzhen Hospital, Capital Medical University, presented a complex clinical application case and real-world follow-up results from multiple centers after CH-VAD became commercially available.

In this reported case, Professor Gong's team successfully performed a combination surgery that included VAD implantation, Bentall, CABG, TVP, and PFO, achieving an ideal long-term clinical outcome. This case demonstrated the feasibility of CH-VAD implantation alongside complex cardiac and major vascular surgeries, confirming the stable, effective, and excellent performance of CH-VAD.
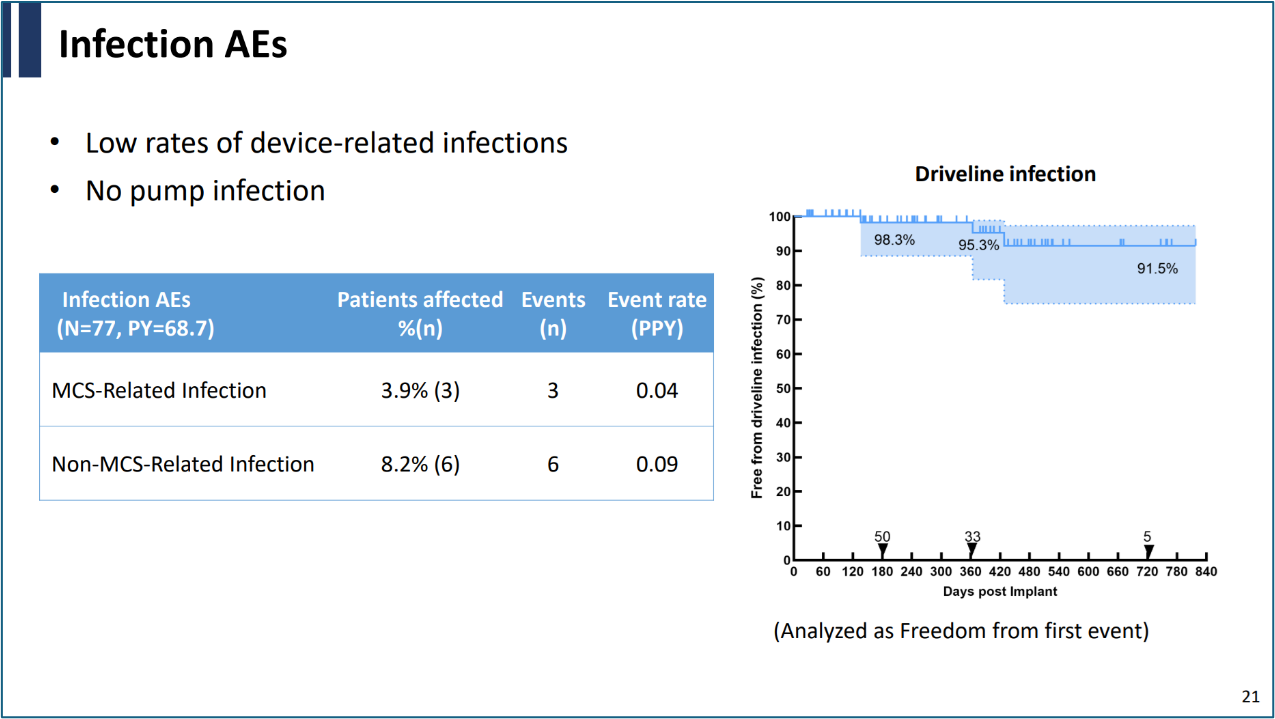
During his presentation, Professor Gong Ming shared the results from a multicenter real-world study conducted after the commercialization of CH-VAD. The participating centers included Beijing Anzhen Hospital, Wuhan Asia Heart Hospital, Sichuan Provincial People's Hospital, Henan Provincial Chest Hospital, Zhongshan Hospital Fudan University, Jiangsu Province Hospital, and Shanghai Chest Hospital, with a total of 77 patients included in the study. The average follow-up duration was nearly 1 year. The data revealed that the survival rates of patients at 6 months and 1 yea reached 91.6%, with no patients required bridge to heart transplantation, no pump failures or pump replacements. The average pump support duration was 320 days, consistently reinforcing the safety and reliability of the product.
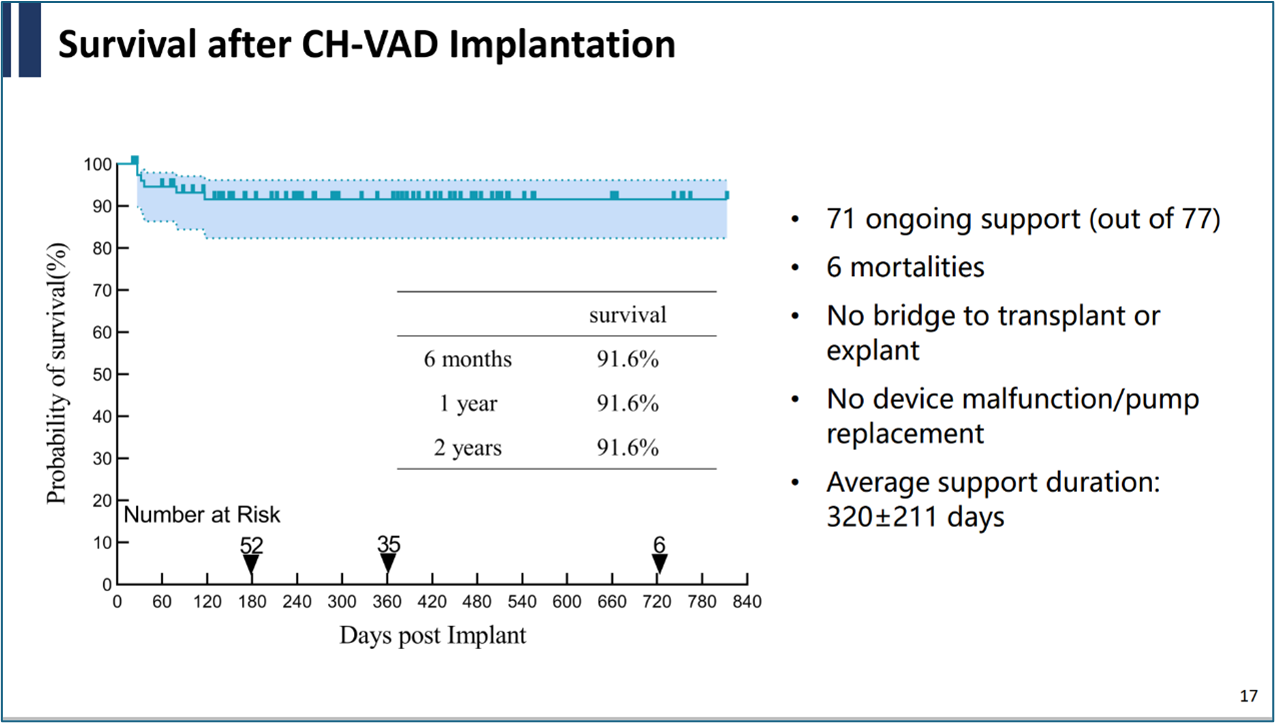
During the follow-up, there were no pump thrombosis incidents, and the incidence of thrombotic or hemorrhagic events was extremely low, further validating the excellent hemocompatibility of CH-VAD.
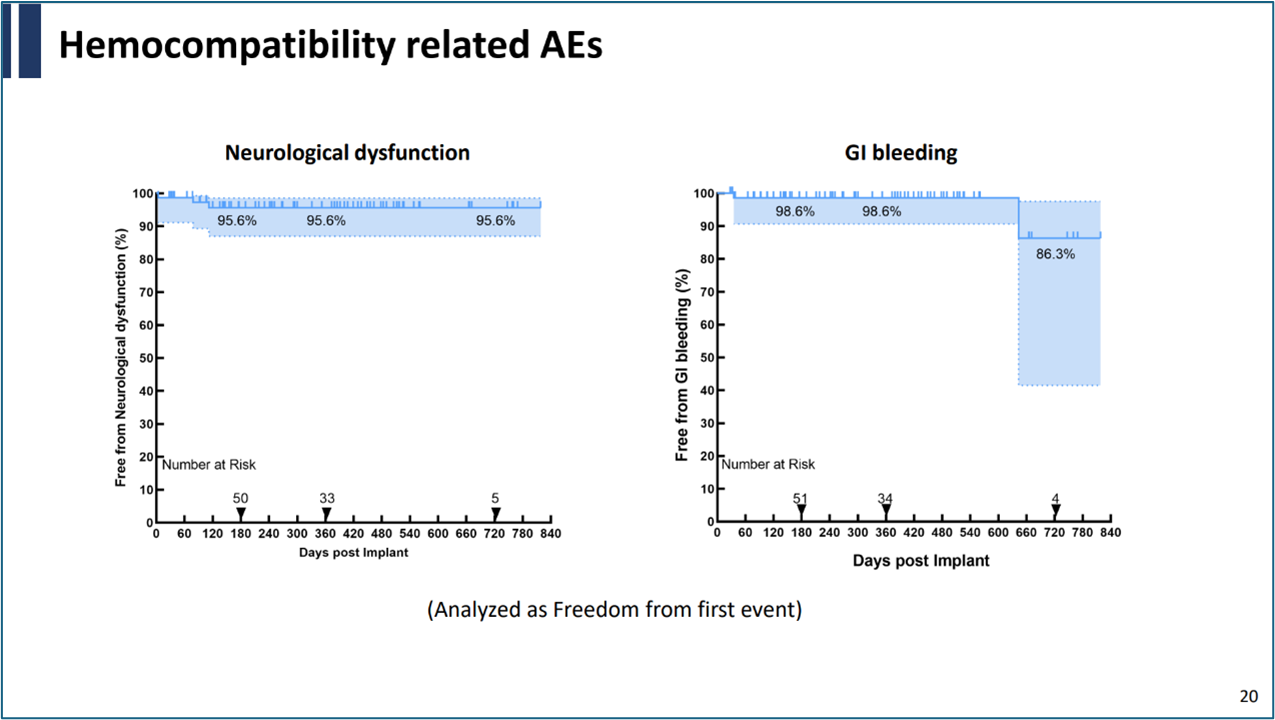
A notable point from the study was the ultra-low incidence of percutaneous cable infections, attributed to the innovative design of the CH-VAD's percutaneous cable. This design significantly improves long-term care convenience and patients' quality of life.
At the conference, Professor Cui Yong from Zhejiang Provincial People's Hospital shared his clinical experience with CH-VAD and presented a modified minimally invasive VAD implantation technique specifically designed for patients undergoing reoperation. Professor Cui outlined several minimally invasive surgical approaches, tailored to different clinical scenarios, to maximize the clinical benefits for patients. The series of cases shared by Professor Cui demonstrated satisfactory short-term and long-term clinical outcomes. He emphasized that minimally invasive VAD implantation can serve as a viable alternative to median sternotomy, particularly for reoperation patients. This approach offers significant clinical benefits, such as reduced surgical trauma, lower blood loss, and faster recovery.

Professor Hua Zhengdong from Wuhan Asia Heart Hospital shared a highly challenging case of fulminant giant cell myocarditis complicated by cardiogenic shock. The patient, a 52-year-old female with a small body size and a left ventricular diameter of only 4.5 cm, suffered from multi-organ dysfunction and had been supported by V-A ECMO for 25 days before undergoing CH-VAD implantation. A short-term right ventricular assist device was implanted concurrently during the surgery. With meticulous multidisciplinary management, the patient gradually recovered organ function and was successfully discharged. Over more than two years of follow-up, she has maintained a high quality of life without any VAD-related complications. Professor Hua emphasized that timely adjustment of circulatory support strategies is critical for the successful treatment of such patients. In this case, CH-VAD proved to be easy to implant, with adequate perfusion, showcasing excellent short-term and long-term efficacy.

At the conference, BrioHealth Technologies organized a simulation-based surgical training session and engaged in extensive and in-depth exchanges with experts from various countries, creating a highly enthusiastic atmosphere. Experts expressed strong interest in the design features and surgical implantation techniques of both CH-VAD and BrioVAD and many conveyed a keen desire to introduce these products to their respective national and regional markets.




As an internationally recognized VAD expert, Dr. Chen Chen, Founder of BrioHealth Technologies, was invited to deliver an academic presentation titled The Pulsatile Performance of a Fully Magnetically Suspended Blood Pump at the ISMCS-JSAO Joint Forum during the ISMCS 2024 conference. In his presentation, Dr. Chen discussed the results of simulated circulatory device tests, which confirmed that under pulsatile conditions, the blood pump's real-time operating point does not move along a static H-Q curve at a constant speed. This insight led to a deeper analysis of the correspondence between dynamic H-Q and different phases of the cardiac cycle. Dr. Chen elaborated on the importance of studying the dynamic H-Q characteristics of blood pumps under pulsatile conditions, and how hemodynamic compatibility plays a critical role in the VAD engineering field and clinical applications. His talk sparked enthusiastic discussion and drew significant attention from attending experts.



Promoting the academic development in the MCS field worldwide is a key component of BrioHealth Technologies' global strategy. For the first time, BrioHealth Technologies presented a series of research achievements at a high-level international MCS conference, further validating the breakthrough technological innovations of its core products and their outstanding long-term clinical efficacy. This participation not only strengthened the company’s international academic standing but also contributed to enhancing China's global academic influence in the MCS field, providing strong momentum to accelerate BrioHealth Technologies' overseas strategy and expand its presence in global markets.
On August 7, People’s Daily published an article titled Domestic Fully Magnetically Levitated VAD Serving Patients, commending CH Biomedical for breaking the international technological monopoly for developing a domestically produced, fully magnetically levitated VAD with independent intellectual property rights. The device is currently available in nearly 40 hospitals across China, enabling over 140 patients with advanced heart failure to embark on a “new life”.
Recently, at the 2022 China Medical Development Conference—widely regarded as the country’s premier medical forum—the Chinese Academy of Medical Sciences (CAMS) released two lists: China’s Major Medical Achievements of the 21st Century and China’s Important Medical Advancements in 2021. As a global innovator in the field of Ventricular Assist Devices (VADs), CH Biomedical’s independently developed CH-VAD (NMPA (A) 20213120987) was included in China’s Important Medical Advancements in 2021.
On November 24th 2021, the implantable CH-VAD® left ventricular assist system (CH-VAD® LVAS) of CH Biomedical was approved by the National Medical Products Administration (NMPA) for marketing (Registration No.: GXZZ 20213120987). CH-VAD LVAS was developed independently by CH Biomedical, it is the first Left Ventricular Assist Device (LVAD) with complete independent intellectual property rights approved by NMPA in China, and the first full magnetic levitation LVAD approved by NMPA.


